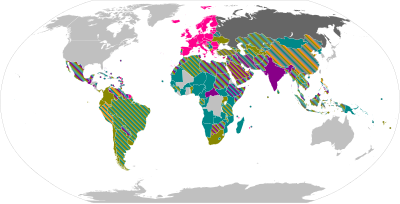
List_of_COVID-19_vaccine_authorizations
National regulatory authorities have granted full or emergency use authorizations for 40 COVID-19 vaccines.
Ten vaccines have been approved for emergency or full use by at least one stringent regulatory authority recognized by the World Health Organization (WHO): Pfizer–BioNTech, Oxford–AstraZeneca, Sinopharm BIBP, Moderna, Janssen, CoronaVac, Covaxin, Novavax, Convidecia, and Sanofi–GSK.[1] Seven others are under assessment by the WHO: Sputnik V, Sinopharm WIBP, Abdala, Zifivax, Corbevax, COVIran Barekat, and SCB-2019.[2]
Of the 40 vaccines, 16 have a full or emergency authorization in only one country, 12 in ten or fewer countries, and 12 in more than ten countries.
Note that in some countries, vaccines may be authorized solely for travel purposes. They may not be approved for the general population. For example, the CoronaVac, Covishield, BBIBP-CorV and Covaxin vaccines are not part of Australia's national vaccination program; however, they are recognized for the purpose of travel to Australia.[3][4][5]
|
|
Full authorization
Emergency authorization
Allowed for travel
Usage stopped | |||
The Oxford–AstraZeneca COVID-19 vaccine, sold under the brand names Vaxzevria[6] and Covishield,[7] is a viral vector vaccine[8] produced by the British University of Oxford, British-Swedish company AstraZeneca, and the Coalition for Epidemic Preparedness Innovations.[8][9][10] Finland, Denmark, and Norway suspended the use of the Oxford–AstraZeneca vaccine due to a small number of reports of a rare blood clot disorder.[11][12][13][14] Slovakia suspended its use after the death of a predisposed recipient.[15] South Africa suspended its use because a small trial found only minimal protection against mild to moderate disease from the locally predominant Beta variant.[16] Japan approved the vaccine for emergency use in May 2021, but did not plan to use them immediately because of rare cases of a blood clotting disorder reported overseas.[17] Later, Japan started to use the vaccine for people aged 40 or over to mitigate the surge of the Delta variant in August.[18] Finland ceased use of the vaccine as the last batch expired on 30 November 2021. Until then it was only offered for those aged 65 or more due to extremely rare coagulation disorders among younger recipients of the vaccine. After this Finland will not procure more of the vaccine.[19][20][21][22] The AstraZeneca vaccine is the most widely accepted internationally,[23] and the most popular in terms of total inoculated worldwide, over 1.3 billion.[24][25][26][27] The AstraZeneca vaccine is administered in more countries than any other vaccine.[28]
- Full (5)
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
- Emergency (170)
- Afghanistan[40][41]
- Albania[42]
- Algeria[43]
- Andorra[44]
- Angola[45]
- Argentina[46]
- Armenia[47]
- Azerbaijan[48]
- Bahrain[49]
- Bangladesh[50][51]
- Benin[48]
- Bhutan[52][53]
- Bolivia[48]
- Bosnia and Herzegovina[54]
- Botswana[55]
- Brunei[56]
- Burkina Faso[57]
- Cambodia[48]
- Cameroon[48]
- Cape Verde[58]
- Central African Republic[57]
- Chile[59]
- Colombia[60]
- Comoros[48]
- Congo-Brazzaville[48]
- Congo-Kinshasa[61]
- Costa Rica[62]
- Djibouti[48]
- Dominican Republic[63]
- East Timor[64]
- Ecuador[65]
- Egypt[66]
- El Salvador[67]
- Eswatini[68]
- Ethiopia[69][70][71]
- Fiji[48]
- Gambia[72]
- Georgia[73]
- Ghana[74]
- Guatemala[75]
- Guinea-Bissau[76]
- Guinea[57]
- Honduras[48]
- Indonesia[77]
- Iran[78]
- Iraq[79]
- Ivory Coast[80]
- Japan[81]
- Jordan[82]
- Kenya[83]
- Kiribati[57]
- Kosovo[84][85]
- Kuwait[86]
- Kyrgyzstan[57]
- Laos[57]
- Lebanon[87]
- Lesotho[88]
- Liberia[89]
- Libya[90][91]
- Madagascar[48]
- Malawi[92][93]
- Malaysia[94]
- Maldives[95]
- Mali[96]
- Mauritania[57]
- Mauritius[97]
- Mexico[98]
- Micronesia[57]
- Moldova[99]
- Mongolia[100]
- Montenegro[48]
- Morocco[101]
- Mozambique[48]
- Myanmar[102]
- Namibia[103]
- Nauru[104]
- Nepal[105]
- New Zealand[57]
- Nicaragua[48]
- Niger[48]
- Nigeria[106]
- North Macedonia[107]
- Oman[48]
- Pakistan[108]
- Palestine[48]
- Panama[48]
- Papua New Guinea[109][110]
- Paraguay[57]
- Peru[111]
- Philippines[112]
- Qatar[57]
- Rwanda[113]
- Samoa[104]
- São Tomé and Príncipe[48]
- Saudi Arabia[114]
- Senegal[57]
- Serbia[115]
- Seychelles[116]
- Sierra Leone[117]
- Singapore[118] (restricted)
- Solomon Islands[48]
- Somalia[119]
- South Korea[120][121]
- South Sudan[122]
- Sri Lanka[123]
- Sudan[124][125]
- Syria[57]
- Taiwan[126]
- Tajikistan[127]
- Thailand[128]
- Togo[129]
- Tonga[130][131][132]
- Tunisia[57]
- Turkmenistan[57]
- Tuvalu[104]
- Uganda[133]
- Ukraine[134]
- United Arab Emirates[48]
- United Kingdom[135][136][137]
- Uruguay[48]
- Uzbekistan[48]
- Vanuatu[57]
- Vietnam[138]
- Yemen[139]
- Zambia[140][141][142]
- Zimbabwe[57]
CARPHA countries and entities[143]
- Antigua and Barbuda
- Bahamas
- Barbados
- Belize
- Dominica
- Grenada
- Guyana
- Haiti
- Jamaica
- Saint Kitts and Nevis
- Saint Lucia
- Saint Vincent and the Grenadines
- Suriname[144]
- Trinidad and Tobago
- Anguilla
- Aruba
- British Virgin Islands
- Bermuda
- Caribbean Netherlands
- Cayman Islands
- Curaçao
- Montserrat
- Sint Maarten
- Turks and Caicos Islands
- Cook Islands[48]
- Falkland Islands[48]
- French Polynesia[48]
- Greenland[145]
- Guadeloupe[57]
- Guernsey[48]
- Isle of Man[48]
- Jersey[48]
- Northern Cyprus[146]
- Pitcairn[57]
- Saint Helena, Ascension and Tristan da Cunha[57]
- Wallis and Futuna[48]
- World Health Organization[147][2][148][149][150][151]
- Travel-only
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
| |||
The Pfizer–BioNTech COVID-19 vaccine, sold under the brand name Comirnaty,[37] is an mRNA vaccine[156] produced by the German company BioNTech and the American company Pfizer.[156][157][158] In Hong Kong, Macau, and Taiwan, Comirnaty is distributed by Fosun Pharma.[159][160][161][162][163][164]
Original
- Full (39)
- Australia[165][166]
- Brazil[167]
- Canada[168][169]
- Marshall Islands[lower-alpha 1][lower-alpha 2]
- Micronesia[lower-alpha 1][lower-alpha 2]
- New Zealand[170]
- Palau[lower-alpha 1][lower-alpha 2]
- Saudi Arabia[173][145]
- Switzerland[174][175]
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
- Emergency (145)
- Afghanistan[48]
- Albania[178]
- Algeria[179]
- Andorra[180]
- Argentina[181]
- Armenia[182]
- Azerbaijan[57]
- Bahrain[183][184]
- Bangladesh[185]
- Benin[57]
- Bhutan[57]
- Bolivia[48]
- Bosnia and Herzegovina[54]
- Botswana[186]
- Brunei[56]
- Cambodia[57]
- Cameroon[57]
- Cape Verde[48]
- Chile[187]
- China (For German citizens)[188]
- Colombia[189]
- Congo-Kinshasa[190]
- Costa Rica[191]
- Djibouti[57]
- Dominican Republic[192]
- East Timor[57]
- Ecuador[193]
- Egypt[57]
- El Salvador[48][194]
- Eswatini[57]
- Fiji[195]
- Gabon[57]
- Georgia[57]
- Ghana[196]
- Guatemala[75]
- Guinea[57]
- Honduras[57]
- Indonesia[197]
- Iraq[198]
- Israel[199]
- Ivory Coast[57]
- Japan[81][200][201]
- Jordan[202]
- Kazakhstan[57]
- Kenya[203]
- Kosovo[57]
- Kuwait[204]
- Kyrgyzstan[57]
- Laos[57]
- Lebanon[205]
- Libya[48]
- Malawi[206]
- Malaysia[207]
- Maldives[208]
- Mexico[209][210]
- Moldova[99]
- Monaco[211]
- Mongolia[212]
- Montenegro[57]
- Morocco[57]
- Namibia[57]
- Nepal[213]
- Nicaragua[57]
- Nigeria[214]
- North Macedonia[215]
- Oman[216]
- Pakistan[217]
- Palestine[48]
- Panama[218]
- Papua New Guinea[219]
- Paraguay[220][221]
- Peru[222]
- Philippines[223]
- Qatar[224]
- Rwanda[48]
- Samoa[57]
- San Marino[48]
- Serbia[225][226]
- Singapore[227]
- Somalia[57]
- South Africa[228][229]
- South Korea[230][231]
- Sri Lanka[232]
- Sudan[48]
- Syria[57]
- Taiwan[163][164]
- Tajikistan[57]
- Tanzania[57]
- Thailand[233]
- Tonga[57]
- Tunisia[145]
- Turkey[234]
- Uganda[57]
- Ukraine[235]
- United Arab Emirates[236]
- United Kingdom[237][238][239]
- Uruguay[240]
- Uzbekistan[241]
- Vatican City[242][243]
- Vietnam[244]
- Yemen[57]
- Zambia[245]
CARPHA countries and entities[246][247]
- Antigua and Barbuda
- Bahamas
- Barbados
- Belize
- Dominica
- Grenada
- Guyana
- Haiti
- Jamaica
- Saint Kitts and Nevis
- Saint Lucia
- Saint Vincent and the Grenadines
- Suriname
- Trinidad and Tobago
- Anguilla
- Aruba
- British Virgin Islands
- Bermuda
- Caribbean Netherlands
- Cayman Islands
- Curaçao
- Montserrat
- Sint Maarten
- Turks and Caicos Islands
- American Samoa[57]
- Cook Islands[57]
- Faroe Islands[248]
- French Polynesia[57]
- Gibraltar[48]
- Greenland[249]
- Guadeloupe[57]
- Guam[57]
- Guernsey[48]
- Isle of Man[48]
- Jersey[48]
- Macau[250]
- Martinique[57]
- New Caledonia[48]
- Niue[57]
- Northern Cyprus[146]
- Northern Mariana Islands[57]
- Puerto Rico[57]
- Tokelau[57]
- World Health Organization[147][2][251][252]
Bivalent original–BA.1
- Japan[citation needed]
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
Bivalent original–BA.4/5
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
- Hong Kong[255]
- Macau[256]
- World Health Organization[147]
XBB.1.5
- United States[257]
Full authorization
Full authorization, not used
Emergency authorization
Allowed for travel
Usage stopped | |||
The Janssen COVID-19 vaccine[258] is a viral vector vaccine[259] produced by Janssen Pharmaceutica (a subsidiary of Johnson & Johnson) and Beth Israel Deaconess Medical Center.[260][261] It is also known as Johnson & Johnson COVID-19 Vaccine and as COVID-19 Vaccine Janssen.[262] Three countries, Denmark, Finland, and Norway, discontinued the use of the Janssen vaccine in favor of other available vaccines due to a possible link between the vaccine and a rare blood clot disorder.[263][19][264] The use of the Janssen adenovirus vector vaccine began in Finland in October 2021. It is only offered for those aged 65 and over because of a very rare risk of thrombosis in younger age groups.[20][22][21] The United States began use of the Janssen vaccine in March 2021,[265] but discouraged use in favor of other available vaccines in December 2021 due to the risk of a rare clotting disorder.[266] The Janssen vaccine became unavailable in the United States in May 2023 after all existing doses expired.[267]
- Full (3)
- Emergency (133)
- Afghanistan[57]
- Andorra[44]
- Argentina[274]
- Bahamas[275]
- Bahrain[276][277]
- Bangladesh[278][279]
- Benin[57]
- Bolivia[57]
- Botswana[280]
- Brazil[281]
- Brunei[57]
- Burkina Faso[57]
- Cambodia[57]
- Cameroon[57]
- Central African Republic[245]
- Chile[282]
- Colombia[283]
- Congo-Kinshasa[190]
- Djibouti[57]
- Egypt[57]
- Eswatini[57]
- Ethiopia[57]
- Gabon[245]
- Ghana[284]
- Guinea[57]
- Honduras[48]
- India[285]
- Indonesia[286]
- Iran[245]
- Iraq[57]
- Ivory Coast[57]
- Jordan[57]
- Kenya[203]
- Kuwait[287]
- Laos[57]
- Lebanon[57]
- Lesotho[57]
- Liberia[245]
- Libya[48]
- Madagascar[57]
- Malawi[57]
- Malaysia[288]
- Maldives[289]
- Mali[245]
- Marshall Islands[lower-alpha 1]
- Mauritania[245]
- Mexico[290][291]
- Micronesia[lower-alpha 1]
- Moldova[292]
- Monaco[57]
- Morocco[57]
- Mozambique[57]
- Namibia[57]
- Nepal[293]
- New Zealand[57]
- Nicaragua[57]
- Nigeria[294]
- Oman[57]
- Pakistan[57]
- Palau[lower-alpha 1]
- Palestine[57]
- Papua New Guinea[57]
- Peru[295]
- Philippines[296]
- Qatar[57]
- Rwanda[57]
- Saudi Arabia[57]
- Senegal[57]
- Singapore[118] (restricted)
- Somalia[57]
- South Africa[297]
- South Korea[298]
- South Sudan[57]
- Sudan[57]
- Syria[57]
- Taiwan (not used)[299]
- Tanzania[57]
- Thailand[300]
- Tunisia[301]
- Uganda[57]
- Ukraine[57]
- United Arab Emirates[57]
- United Kingdom[302]
- United States[265][303] (no longer available as of May 7, 2023 [267])
- Vanuatu[57]
- Vietnam[304][305]
- Yemen[57]
- Zambia[140][141][142]
- Zimbabwe[306]
EMA countries[262][38][307][1]
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
CARPHA countries and entities[308]
- Antigua and Barbuda
- Bahamas
- Barbados
- Belize
- Dominica
- Grenada
- Guyana
- Haiti
- Jamaica
- Saint Kitts and Nevis
- Saint Lucia
- Saint Vincent and the Grenadines[309]
- Suriname
- Trinidad and Tobago
- Anguilla
- Aruba
- British Virgin Islands
- Bermuda
- Caribbean Netherlands
- Cayman Islands
- Curaçao
- Montserrat
- Sint Maarten
- Turks and Caicos Islands
Non-country entities
- American Samoa[57]
- French Polynesia[57]
- Greenland[145]
- Guam[57]
- New Caledonia[57]
- Northern Cyprus[310]
- Northern Mariana Islands[57]
- Puerto Rico[57]
- Africa Regulatory Taskforce[311]
- World Health Organization[147][312][313]
- Travel-only
- Hong Kong[152]
- Japan[314]
- Turkey[315][unreliable source?]
| |||
The Moderna COVID-19 vaccine, also known as Spikevax,[316] is an mRNA vaccine[317] produced by the American company Moderna, the U.S. National Institute of Allergy and Infectious Diseases, the U.S. Biomedical Advanced Research and Development Authority, and the Coalition for Epidemic Preparedness Innovations.[318][319] The Moderna vaccine is not offered for men under 30 years of age in Finland as a precaution to reduce a very rare risk of myocarditis.[320][22]
Original
- Full (34)
EMA countries[316][38][328][1]
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
- Emergency (116)
- Andorra[44]
- Argentina[329]
- Armenia[57]
- Bahrain[57]
- Bangladesh[330]
- Bhutan[57]
- Botswana[331]
- Bolivia[332]
- Brazil[333]
- Brunei[57]
- Cape Verde[57]
- Chile[334]
- Colombia[335]
- Congo-Brazzaville[48]
- Congo-Kinshasa[190]
- Djibouti[57]
- Egypt[336]
- El Salvador[57]
- Fiji[57]
- Ghana[337]
- Guatemala[75]
- Honduras[338]
- India[339]
- Indonesia[340]
- Iran[57]
- Iraq[57]
- Israel[341]
- Japan[81][342]
- Jordan[57]
- Kenya[343]
- Kuwait[344]
- Kyrgyzstan[57]
- Lebanon[57]
- Libya[48]
- Malawi[206]
- Malaysia[345]
- Maldives[346]
- Marshall Islands[lower-alpha 1]
- Mexico[347][348]
- Micronesia[lower-alpha 1]
- Moldova[292]
- Monaco[57]
- Mongolia[100]
- Morocco[57]
- Nepal[349]
- Nigeria[350]
- Oman[57]
- Pakistan[57]
- Palau[lower-alpha 1]
- Palestine[48]
- Paraguay[57]
- Peru[351]
- Philippines[352]
- Qatar[353]
- Rwanda[48]
- Saudi Arabia[114]
- Serbia[354]
- Singapore[355]
- South Korea[356]
- Sri Lanka[57]
- Sudan[57]
- Syria[57]
- Taiwan[357]
- Tajikistan[358]
- Thailand[359]
- Tunisia[57]
- Uganda[57]
- Ukraine[360]
- United Arab Emirates[361]
- Uzbekistan[362]
- Vietnam[363][364]
- Yemen[57]
CARPHA countries and entities[246][308]
- Antigua and Barbuda
- Bahamas
- Barbados
- Belize
- Dominica
- Grenada
- Guyana
- Haiti
- Jamaica
- Saint Kitts and Nevis
- Saint Lucia
- Saint Vincent and the Grenadines
- Suriname
- Trinidad and Tobago
- Anguilla
- Aruba
- British Virgin Islands
- Bermuda
- Caribbean Netherlands
- Cayman Islands
- Curaçao
- Montserrat
- Sint Maarten
- Turks and Caicos Islands
Non-country entities
- American Samoa[57]
- Faroe Islands[48]
- Greenland[48]
- Guadeloupe[57]
- Guam[57]
- Guernsey[48]
- Isle of Man[57]
- Jersey[48]
- Northern Mariana Islands[57]
- Puerto Rico[57]
- Wallis and Futuna[57]
- World Health Organization[147][365][366]
- Travel-only
Bivalent original–BA.1
- Australia[368]
- Canada[322][369]
- Japan[citation needed]
- Singapore[370]
- Switzerland[371]
- Taiwan[372][373]
- United Kingdom[374]
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
Bivalent original–BA.4/5
- Taiwan[376]
XBB.1.5
- United States[377]
| |||
The Sinopharm BIBP COVID-19 vaccine is an inactivated virus vaccine produced by the China National Pharmaceutical Group (Sinopharm) and its Beijing Institute of Biological Products.[378][379]
- Full (4)
- Emergency (111)
- Afghanistan[385]
- Algeria[386]
- Angola[387]
- Argentina[388]
- Armenia[57]
- Bangladesh[389]
- Belarus[390]
- Bhutan[391]
- Bolivia[392]
- Bosnia and Herzegovina[393]
- Brazil[333]
- Brunei[394]
- Burkina Faso[57]
- Burundi[57]
- Cambodia[395]
- Cameroon[48]
- Cape Verde[57]
- Chad[396]
- Comoros[48]
- Congo-Brazzaville[48]
- Cuba[397]
- Djibouti[48]
- Dominican Republic[398]
- Egypt[399]
- El Salvador[57]
- Equatorial Guinea[400]
- Ethiopia[401]
- Gabon[402]
- Gambia[57]
- Georgia[403]
- Guinea-Bissau[48]
- Guinea[57]
- Hungary[404][405]
- Indonesia[406]
- Iran[407]
- Iraq[79]
- Ivory Coast[57]
- Jordan[408]
- Kazakhstan[409]
- Kenya[343]
- Kiribati[57]
- Kuwait[48]
- Kyrgyzstan[410]
- Laos[411]
- Lebanon[412]
- Lesotho[57]
- Libya[413]
- Madagascar[57]
- Malawi[414]
- Malaysia[415]
- Maldives[416]
- Mauritania[417]
- Mauritius[48]
- Mexico[418][419]
- Moldova[420]
- Mongolia[421]
- Montenegro[422]
- Morocco[423]
- Mozambique[424]
- Myanmar[425]
- Namibia[426]
- Nepal[427]
- Nicaragua[428]
- Niger[429]
- Nigeria[430]
- North Korea[431]
- North Macedonia[432]
- Oman[48]
- Pakistan[433]
- Palestine[434]
- Papua New Guinea[435]
- Paraguay[57]
- Peru[436]
- Philippines[437]
- Qatar[48]
- Rwanda[438]
- Senegal[439]
- Serbia[440]
- Sierra Leone[441]
- Singapore[442]
- Solomon Islands[443]
- Somalia[444]
- South Sudan[445]
- Sri Lanka[446]
- Sudan[447][448]
- Syria[57]
- Tanzania[57]
- Thailand[449]
- Tunisia[450]
- Turkmenistan[451]
- Vanuatu[452]
- Venezuela[453]
- Vietnam[454]
- Yemen[48]
- Zambia[455]
- Zimbabwe[456]
CARPHA countries and entities[457]
- Antigua and Barbuda
- Bahamas
- Barbados
- Belize
- Dominica
- Grenada
- Guyana
- Haiti
- Jamaica
- Saint Kitts and Nevis
- Saint Lucia
- Saint Vincent and the Grenadines
- Suriname
- Trinidad and Tobago
- Anguilla
- Aruba
- British Virgin Islands
- Bermuda
- Caribbean Netherlands
- Cayman Islands
- Curaçao
- Montserrat
- Sint Maarten
- Turks and Caicos Islands
- Travel-only
- Andorra[461]
- Australia[462][463]
- Austria[464] (only for entry)
- Bulgaria[465]
- Canada[466]
- Chile[467]
- Costa Rica[468]
- Croatia[469]
- Cyprus[470]
- Czech Republic[471] (only for Hungarian citizens and EU nationals vaccinated in Hungary)
- Estonia[472] (only if approved in a person's country of origin)
- Finland[473]
- Greece[474]
- Grenada[475]
- Hong Kong[152]
- Iceland[476]
- Ireland[477]
- Japan[314]
- Latvia[478]
- Liechtenstein[479]
- New Zealand[367]
- Oman[480]
- Panama[481]
- Portugal[482] (only in Madeira)
- Qatar[483]
- Saudi Arabia (for short visits, Hajj and Umrah only)[484]
- Slovakia[485]
- Slovenia[486]
- South Korea[487]
- Spain[488]
- St. Kitts and Nevis[489]
- St. Lucia[490]
- St. Vincent and the Grenadines[491]
- Sweden[492]
- Switzerland[493]
- The Netherlands[494]
- Turkey[495]
- Ukraine[496]
- United Kingdom[497]
- United States[498]
- Uruguay[499] (only if approved in a person's country of origin)
Full authorization
Emergency authorization
Authorization expired
Allowed for travel
Rejected | |||
The Sputnik V COVID-19 vaccine is a viral vector vaccine[500] produced by the Russian Gamaleya Research Institute of Epidemiology and Microbiology.
- Full (3)
- Emergency (76)
- Albania[42]
- Algeria[506]
- Angola[507]
- Antigua and Barbuda[508]
- Argentina[509]
- Armenia[510]
- Azerbaijan[511][512]
- Bahrain[513]
- Bangladesh[514]
- Belarus[515]
- Bolivia[516]
- Bosnia and Herzegovina[517]
- Brazil[518] (restricted)
- Cambodia[519]
- Cameroon[520]
- Chile[521]
- Congo-Brazzaville[507]
- Djibouti[507]
- Ecuador[522]
- Egypt[66]
- Gabon[523]
- Gambia[524]
- Ghana[525]
- Guatemala[526]
- Guinea[527]
- Guyana[528]
- Honduras[529]
- Hungary[530][517][405]
- India[531]
- Indonesia[532]
- Iran[533]
- Iraq[534]
- Ivory Coast[57]
- Jordan[535]
- Kazakhstan[536]
- Kenya[537]
- Kyrgyzstan[538]
- Laos[411]
- Lebanon[539]
- Libya[90][91]
- Maldives[540]
- Mali[541]
- Mauritius[542]
- Mexico[543]
- Moldova[99]
- Mongolia[544]
- Montenegro[545]
- Morocco[546]
- Myanmar[547]
- Namibia[548]
- Nepal[549]
- Nicaragua[550]
- Nigeria[551]
- North Macedonia[552]
- Oman[553]
- Pakistan[554]
- Palestine[555]
- Panama[556]
- Paraguay[557]
- Peru[558]
- Philippines[559]
- Rwanda[57]
- Saint Vincent and the Grenadines[309]
- San Marino[560][561]
- Serbia[562]
- Seychelles[563]
- Sri Lanka[564]
- Syria[565]
- Tajikistan[57]
- Tanzania[566]
- Tunisia[567]
- Turkey (limited use)[568][569]
- United Arab Emirates[570]
- Venezuela[571]
- Vietnam[572]
- Zimbabwe[573]
| |||
The CoronaVac COVID-19 vaccine is an inactivated virus vaccine[590] produced by the Chinese company Sinovac Biotech.[590][591][592]
- Full (1)
- China[593]
- Emergency (71)
- Afghanistan[48]
- Albania[42]
- Algeria[57]
- Argentina[245]
- Armenia[595]
- Azerbaijan[596]
- Bangladesh[597]
- Benin[598]
- Bolivia[599]
- Bosnia and Herzegovina[600]
- Botswana[601]
- Brazil[602]
- Cambodia[603]
- Chile[604]
- Colombia[605]
- Djibouti[606]
- Dominica[145]
- Dominican Republic[607]
- East Timor[608]
- Ecuador[609]
- Egypt[610]
- El Salvador[611]
- Equatorial Guinea[57]
- Fiji[612]
- Gabon[145]
- Georgia[613]
- Guinea[614]
- Guyana[145]
- Hungary[57]
- Indonesia[615][616]
- Iraq[48]
- Jordan[48]
- Kazakhstan[617]
- Kuwait[48]
- Laos[593]
- Lebanon[48]
- Libya[618]
- Malawi[414]
- Malaysia[619]
- Maldives[57]
- Mexico[620]
- Moldova[621]
- Morocco[145]
- Myanmar[622]
- Nepal[623]
- North Macedonia[624]
- Oman[553]
- Pakistan[625]
- Palestine[57]
- Panama[626]
- Paraguay[627]
- Philippines[628]
- Qatar[48]
- Rwanda[57]
- Saint Vincent and the Grenadines[145]
- Saudi Arabia[629]
- Singapore[630]
- Somalia[48]
- South Africa[631]
- Sri Lanka[632]
- Sudan[48]
- Syria[57]
- Tajikistan[633]
- Tanzania[634]
- Thailand[635]
- Togo[636]
- Trinidad and Tobago[637]
- Tunisia[638]
- Turkey[639]
- Turkmenistan[640]
- Uganda[57]
- Ukraine[641]
- United Arab Emirates[48]
- Uruguay[593]
- Uzbekistan[642]
- Venezuela[643]
- Yemen[57]
- Zimbabwe[573]
- Travel-only
- Andorra[461]
- Australia[462][647]
- Austria[464] (only for entry)
- Canada[466]
- Costa Rica[468]
- Croatia[469]
- Cyprus[470]
- Estonia[472] (only if approved in a person's country of origin)
- Finland[473]
- Greece[474]
- Grenada[475]
- Iceland[476]
- Ireland[477]
- Japan[314]
- Liechtenstein[479]
- Norway[648]
- New Zealand[367]
- Norway[649]
- Palau[650]
- Panama[481]
- Portugal[482] (only in Madeira)
- Slovakia[485]
- Slovenia[486]
- South Korea[487]
- Spain[488]
- St. Kitts and Nevis[489]
- St. Lucia[490]
- St. Vincent and the Grenadines[491]
- Sweden[492]
- Switzerland[493]
- The Netherlands[494]
- Ukraine[496]
- United Arab Emirates[651]
- United Kingdom[497]
- United States[498]
- Vietnam[652]
| |||
The Novavax COVID-19 vaccine, sold under the brand names Nuvaxovid and Covovax, is a subunit COVID-19 vaccine candidate developed by Novavax and the Coalition for Epidemic Preparedness Innovations.[653][654]
- Full (4)
- Emergency (57)
- Bangladesh[660]
- India[661]
- Indonesia[662]
- Monaco[57]
- New Zealand[663]
- Philippines[664]
- Singapore[665]
- South Africa[245]
- Switzerland[666]
- Thailand[667]
- Taiwan[668]
- United Kingdom[669]
- United States[670][671]
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
CARPHA countries and entities[245][673]
- Antigua and Barbuda
- Bahamas
- Barbados
- Belize
- Dominica
- Grenada
- Guyana
- Haiti
- Jamaica
- Saint Kitts and Nevis[489]
- Saint Lucia[490]
- Saint Vincent and the Grenadines[491]
- Suriname
- Trinidad and Tobago
- Anguilla
- Aruba
- British Virgin Islands
- Bermuda
- Caribbean Netherlands
- Cayman Islands
- Curaçao
- Montserrat
- Sint Maarten
- Turks and Caicos Islands
- Travel-only
| |||
Covaxin is an inactivated virus vaccine produced by the Indian company Bharat Biotech in collaboration with the Indian Council of Medical Research–National Institute of Virology.
- Full (1)
- India[34]
- Emergency (50)
- Afghanistan[48]
- Bahrain[48][676]
- Botswana[48][677]
- Central African Republic[48]
- Comoros[48]
- Egypt[48]
- Ethiopia[48][57]
- Guatemala[677] (not used)[678]
- Iran[48][679]
- Iraq[48]
- Jordan[48]
- Kuwait[48]
- Lebanon[48]
- Libya[48]
- Malaysia[680]
- Mauritius[681]
- Mexico[682] (not used)[678]
- Morocco[48]
- Myanmar[683]
- Nepal[48][684]
- Nicaragua[677] (not used)[678]
- Oman[48]
- Pakistan[48]
- Paraguay[48][685][686]
- Philippines[687][688]
- Qatar[48]
- Somalia[48]
- Sudan[48]
- Syria[48]
- Tunisia[48]
- United Arab Emirates[48]
- Venezuela[677] (not used)[678]
- Vietnam[689]
- Yemen[48]
- Zimbabwe[690]
CARPHA countries and entities[245][673]
- Antigua and Barbuda
- Bahamas
- Barbados
- Belize
- Dominica
- Grenada[475]
- Guyana[677] (not used)[678]
- Haiti
- Jamaica
- Saint Kitts and Nevis[489]
- Saint Lucia[490]
- Saint Vincent and the Grenadines[491]
- Suriname
- Trinidad and Tobago[637]
- Anguilla
- Aruba
- British Virgin Islands
- Bermuda
- Caribbean Netherlands
- Cayman Islands
- Curaçao
- Montserrat
- Sint Maarten
- Turks and Caicos Islands
- Travel-only
- Andorra[461]
- Australia[462][463]
- Austria[464] (only for entry)
- Canada[466]
- Costa Rica[468]
- Croatia[469]
- Cyprus[470]
- Estonia[472] (only if approved in a person's country of origin)
- Finland[473]
- Greece[474]
- Hong Kong[152]
- Iceland[476]
- Ireland[477]
- Japan[314]
- Kyrgyzstan[681]
- Liechtenstein[479]
- Mongolia[681]
- New Zealand[367]
- Norway[649]
- Oman[480]
- Palestine[681]
- Palau[650]
- Panama[481]
- Portugal[482] (only in Madeira)
- Saudi Arabia[693]
- Singapore[118]
- Slovakia[485]
- Slovenia[486]
- South Korea[487]
- Spain[488]
- Sri Lanka[694]
- Sweden[492]
- Switzerland[493]
- Thailand[695]
- The Netherlands[494]
- Turkey[495]
- Ukraine[496]
- United Kingdom[497]
- United States[498]
Full authorization
Emergency authorization
Allowed for travel | |||
VLA2001 is an inactivated vaccine developed by Valneva SE and Dynavax Technologies.
- Full (1)
- United Kingdom[696]
- Emergency (32)
- Austria
- Belgium
- Bulgaria
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Estonia
- Finland
- France
- Germany
- Greece
- Hungary
- Iceland
- Ireland
- Italy
- Latvia
- Liechtenstein
- Lithuania
- Luxembourg
- Malta
- Netherlands
- Norway
- Poland
- Portugal
- Romania
- Slovakia
- Slovenia
- Spain
- Sweden
- Travel-only
The Sanofi–GSK COVID-19 vaccine, sold under the brand name VidPrevtyn Beta, is a subunit vaccine developed by Sanofi Pasteur and GSK plc. It is based on the SARS-CoV-2 Beta variant.
Full authorization
Emergency authorization
Allowed for travel | |||
Sputnik Light is a viral vector vaccine,[701] produced by the Russian Gamaleya Research Institute of Epidemiology and Microbiology. It consists of the first dose of the Sputnik V vaccine, which is based on the Ad26 vector.[702]
- Full (0)
- Emergency (28)
- Angola[703]
- Argentina[704]
- Armenia[705]
- Bahrain[706]
- Belarus[707]
- Benin[708]
- Cambodia[519]
- Congo-Brazzaville[709]
- Egypt[710]
- Iran[245]
- India[711]
- Kazakhstan[712]
- Kyrgyzstan[713]
- Laos[57]
- Mauritius[714]
- Mongolia[715]
- Nicaragua[716]
- Palestine[717]
- Philippines[718]
- Russia[719]
- Saint Vincent and the Grenadines[57]
- San Marino[720]
- Syria[721]
- Tanzania[245]
- Tunisia[245]
- Turkmenistan[245][722]
- United Arab Emirates[723]
- Venezuela[724]
Non-country entities
- Travel-only
Full authorization
Emergency authorization
Allowed for travel | |||
Convidecia is a viral vector vaccine[727] produced by the Chinese company CanSino Biologics and the Beijing Institute of Biotechnology of the Academy of Military Medical Sciences.
- Full (1)
- China[728]
- Emergency (9)
- Argentina[729]
- Chile[730]
- Ecuador[731]
- Hungary[732][405]
- Indonesia[286]
- Malaysia[733]
- Mexico[620]
- Moldova[292]
- Pakistan[734]
- Travel-only
Full authorization
Emergency authorization
Allowed for travel | |||
The Sinopharm WIBP COVID-19 vaccine is an inactivated virus vaccine produced by the China National Pharmaceutical Group (Sinopharm) and its Wuhan Institute of Biological Products.
- Full (1)
- China[736]
- Emergency (5)
- Travel-only
Full authorization
Emergency authorization
Allowed for travel | |||
Abdala is a subunit vaccine developed by the Center for Genetic Engineering and Biotechnology in Cuba.
- Full (0)
- Emergency (6)
- Cuba[738][739]
- Mexico[740]
- Nicaragua[741]
- St. Vincent and the Grenadines[742][743]
- Venezuela[744]
- Vietnam[145]
- Travel-only
Full authorization
Emergency authorization
Allowed for travel | |||
EpiVacCorona is a peptide vaccine produced by the Russian State Research Center of Virology and Biotechnology VECTOR.[749]
- Full (1)
- Emergency (4)
- Travel-only
Full authorization
Emergency authorization
Allowed for travel | |||
Zifivax is a subunit vaccine produced by the Chinese company Anhui Zhifei Longcom Biopharmaceutical.[755]
- Full (0)
- Emergency (5)
- Travel-only
Full authorization
Emergency authorization
Allowed for travel | |||
Soberana 02, is a conjugate vaccine developed by the Finlay Institute in Cuba.[761]
- Full (0)
- Emergency (4)
- Travel-only
Full authorization
Emergency authorization
Allowed for travel | |||
CoviVac is an inactivated virus vaccine[701] produced by the Chumakov Centre at the Russian Academy of Sciences.[765]
- Full (0)
- Emergency (3)
- Travel-only
Full authorization
Emergency authorization
Allowed for travel | |||
QazCovid-in, also known as QazVac, is an inactivated virus vaccine developed by the Research Institute for Biological Safety Problems in Kazakhstan.[768]
- Full (0)
- Emergency (2)
- Travel-only
Full authorization
Emergency authorization
Allowed for travel | |||
Minhai COVID-19 vaccine, is an inactivated virus vaccine developed by Minhai Biotechnology Co. and Shenzhen Kangtai Biological Products Co. Ltd. in China.[772]
- Full (0)
- Emergency (2)
- Travel-only
Full authorization
Emergency authorization
Allowed for travel | |||
MVC-COV1901, is a protein subunit vaccine developed by Taiwan's Medigen Vaccine Biologics and Dynavax Technologies.[774]
- Full (0)
- Emergency (3)
- Non-country entities
1. Somaliland[776]
- Travel-only
Corbevax is a protein subunit vaccine developed by Texas Children's Hospital at the Baylor College of Medicine in Houston, Texas, and licensed to Indian biopharmaceutical firm Biological E. Limited (BioE) for development and production.[661][780]
- Full (0)
- Emergency (2)
- Travel-only
COVIran Barekat, is an inactivated virus vaccine developed by Shifa Pharmed Industrial Co. in Iran.[782]
- Full (0)
- Emergency (2)
- Travel-only
Chinese Academy of Medical Sciences COVID-19 vaccine, is an inactivated virus vaccine developed by Chinese Academy of Medical Sciences.[786]
- Full (0)
- Emergency (1)
- China[786]
- Travel-only
ZyCoV-D, is a DNA plasmid based COVID-19 vaccine developed by the Indian pharmaceutical company Cadila Healthcare with support from the Biotechnology Industry Research Assistance Council.[787]
- Full (0)
- Emergency (1)
- India[787]
- Travel-only
FAKHRAVAC (or MIVAC), is an inactivated virus vaccine developed in Iran by the Organization of Defensive Innovation and Research, an organization of Iran's Ministry of Defense.[788]
- Full (0)
- Emergency (1)
- Iran[788]
- Travel-only
COVAX-19, also known as SpikoGen, is a protein subunit vaccine jointly developed by Australian-based company Vaxine and Iran-based company CinnaGen.[789]
- Full (0)
- Emergency (1)
- Iran[789]
- Travel-only
Razi Cov Pars is a protein subunit vaccine developed by Razi Vaccine and Serum Research Institute.[790]
- Full (0)
- Emergency (1)
- Iran[790]
- Travel-only
Turkovac is an inactivated vaccine developed by Health Institutes of Turkey and Erciyes University.[791]
- Full (0)
- Emergency (1)
- Turkey[791]
- Travel-only
Sinopharm CNBG COVID-19 vaccine (NVSI) is a recombinant protein subunit vaccine developed by the National Vaccine & Serum Institute (NVSI, 中生研究院), a subsidiary of China National Biotec Group (CNBG), which in turn is a subsidiary of Sinopharm.[792]
- Full (0)
- Emergency (1)
- United Arab Emirates[792]
- Travel-only
Soberana Plus is a single-dose of conjugate vaccine developed by the Finlay Institute in Cuba.[793]
- Full (0)[793]
- Emergency (2)
- Travel-only
CoVLP is a virus-like particle vaccine grown in an Australian weed, Nicotiana benthamiana. It was developed by Medicago, and is marketed under the name Covifenz Archived 24 February 2022 at the Wayback Machine.
- Full (1)
- Canada[796]
- Emergency (0)
- Travel-only
Noora is a protein-based vaccine developed by the Baqiyatallah University of Medical Sciences.
- Full (0)
- Emergency (1)
- Iran[797]
- Travel-only
SKYCovione is a protein subunit vaccine developed by SK Bioscience.
- Full (0)
- Emergency (1)
- South Korea[798]
Walvax COVID-19 vaccine is an RNA vaccine developed by Walvax Biotechnology, Suzhou Abogen Biosciences, and the PLA Academy of Military Science.[799]
- Full (0)
- Emergency (1)
- Indonesia[800]
iNCOVACC, also called BBV154 is an adenovirus vector vaccine developed by Bharat Biotech, Precision Virologics, and Washington University School of Medicine.
- Full (0)
- Emergency (1)
Gemcovac, or GEMCOVAC-19, is a self-amplifying mRNA vaccine manufactured by Gennova Biopharmaceuticals.
- Full (0)
- Emergency (1)
V-01 is a protein subunit vaccine developed by Livzon Mabpharm.
- Full (0)
- Emergency (1)
IndoVac is a protein subunit vaccine developed by Indonesian pharmaceutical company Bio Farma and Baylor College of Medicine.
- Full (0)
- Emergency (1)
- Indonesia[245]
- via United States authorization through Compact of Free Association[171][172]
- "COVID-19 vaccines: authorised". European Medicines Agency (EMA). 11 January 2021. Retrieved 31 October 2022.
- "International COVID-19 vaccines recognised by Australia". Therapeutic Goods Administration. Australian Government Department of Health. Retrieved 12 January 2022.
- "COVID-19 and the border - Vaccinated travellers". Department of Home Affairs. Australian Government Department of Home Affairs. Archived from the original on 14 March 2022. Retrieved 12 January 2022.
- "COVID-19 vaccinations". Smartraveller. Australian Government Department of Foreign Affairs and Trade. Retrieved 12 January 2022.
- "Vaxzevria (previously COVID-19 Vaccine AstraZeneca) EPAR". European Medicines Agency (EMA). 25 January 2021.
- "Covishield and Covaxin: What we know about India's Covid-19 vaccines". BBC News. 4 March 2021. Archived from the original on 7 March 2021. Retrieved 8 March 2021.
- "Investigating a Vaccine Against COVID-19". ClinicalTrials.gov. United States National Library of Medicine. 26 May 2020. NCT04400838. Archived from the original on 11 October 2020. Retrieved 14 July 2020.
- "A Phase 2/3 study to determine the efficacy, safety and immunogenicity of the candidate Coronavirus Disease (COVID-19) vaccine ChAdOx1 nCoV-19". EU Clinical Trials Register. European Union. 21 April 2020. EudraCT 2020-001228-32. Archived from the original on 5 October 2020. Retrieved 3 August 2020.
- O'Reilly P (26 May 2020). "A Phase III study to investigate a vaccine against COVID-19". ISRCTN. doi:10.1186/ISRCTN89951424. ISRCTN89951424.
- "THL temporarily suspends the use of the AstraZeneca COVID-19 vaccine - Press release - THL". Finnish Institute for Health and Welfare (THL), Finland. Retrieved 5 December 2021.
- "Denmark continues its vaccine rollout without the COVID-19 vaccine from AstraZeneca". Danish Health Authority. 14 April 2021. Retrieved 31 July 2021.
That is why it is important to emphasise that it is still an approved vaccine.
- Peltier E (14 April 2021). "Denmark says it's permanently stopping use of the AstraZeneca vaccine". The New York Times.
- omsorgsdepartementet Ho (12 May 2021). "AstraZeneca-vaksinen tas ut av koronavaksinasjons-programmet". Regjeringen.no (in Norwegian). Retrieved 12 May 2021.
- "Slovakia suspends use of AstraZeneca COVID-19 vaccine as a recipient dies". Reuters. 11 May 2021. Retrieved 23 June 2021.
- "South Africa sells AstraZeneca COVID-19 vaccines to other African countries". Reuters. 21 March 2021. Retrieved 23 June 2021.
- "Japan to donate millions more AstraZeneca vaccine doses across Asia". Kyodo News. 25 June 2021.
- "Japan to give COVID-19 emergency areas AstraZeneca vaccine". The Japan Times. 4 August 2021. Archived from the original on 28 August 2021. Retrieved 28 August 2021.
- "Suspension of AstraZeneca COVID-19 vaccines for those under the age of 65 extended – THL evaluating how it affects the vaccination programme - Press release - THL". Finnish Institute for Health and Welfare (THL), Finland. Retrieved 5 December 2021.
- "Use of AstraZeneca coronavirus vaccine to end in Finland in November - News - THL". Finnish Institute for Health and Welfare (THL), Finland. Retrieved 5 December 2021.
- "Adenovirus vaccines: FAQ - THL". Finnish Institute for Health and Welfare (THL), Finland. Retrieved 3 December 2021.
- "Getting vaccinated against COVID-19: how, why and when? - THL". Finnish Institute for Health and Welfare (THL), Finland. Retrieved 3 December 2021.
- "Which covid-19 vaccine is the most widely accepted for international travel?". The Economist. 23 July 2021. Retrieved 11 June 2022.
- "India celebrates 1 billion COVID-19 vaccine doses with song and dance". Reuters. 21 October 2021. Retrieved 11 June 2022.
- "Oxford vaccine reaches one billion doses released". University of Oxford. 29 July 2021. Retrieved 11 June 2022.
- "Two billion doses of AstraZeneca's COVID-19 vaccine supplied to countries across the world less than 12 months after first approval". AstraZeneca. 16 November 2021. Retrieved 11 June 2022.
- "Vaxzevria COVID-19 Vaccine (AstraZeneca)". precisionvaccinations.com. 8 June 2022. Retrieved 11 June 2022.
- "Where are Vaccines (Company and Brand) Administered Globally?". CDC Covid Data Tracker. 9 June 2022. Retrieved 13 June 2022.
- "AstraZeneca coronavirus vaccine approved for use in Australia by TGA". ABC News (Australia). 16 February 2021. Retrieved 16 February 2021.
- "TGA provisionally approves AstraZeneca's COVID-19 vaccine". Therapeutic Goods Administration (TGA). 16 February 2021. Retrieved 16 February 2021.
- "Brazil grants full approval to Oxford vaccine, orders Sputnik". Brasilia: France 24. Agence France-Presse. 12 March 2021. Retrieved 13 March 2021.
- "AstraZeneca Vaxzevria COVID-19 vaccine". Health Canada. 7 June 2022. Retrieved 30 October 2022.
- "Regulatory Decision Summary - Vaxzevria". Health Canada. Retrieved 26 November 2021.
- Sharma M, Mordani S (27 January 2022). "Covaxin, Covishield granted market approval in India but conditions apply". India Today. Retrieved 29 January 2022.
- "Israel to offer AstraZeneca vaccines starting Thursday". The Times of Israel. 18 October 2021. Retrieved 13 June 2022.
- "Vaccines "Three vaccines have been approved for use in Israel for protection from coronavirus, by Pfizer, Moderna and AstraZeneca."". Vaccines - Corona Traffic Light Model (Ramzor) Website. 31 May 2022. Retrieved 13 June 2022.
- "Comirnaty EPAR". European Medicines Agency (EMA). Retrieved 23 December 2020.
- "Marketing authorisation". European Medicines Agency (EMA). Retrieved 23 March 2021.
- "Comirnaty". Union Register of medicinal products. 21 December 2020. Retrieved 26 November 2021.
- Sediqi AQ (7 February 2021). "First doses of COVID-19 vaccine arrive in Afghanistan from India". Reuters. Retrieved 24 February 2021.
- "India donates 500,000 COVID vaccines to Afghanistan". Al Jazeera. Retrieved 24 February 2021.
- Semini L. "Albania starts mass COVID vaccinations before tourist season". ABC News. Retrieved 29 March 2021.
- Kreo. "البلاد الوطني / وزارة الصناعات الصيدلانية ترخص باستعمال لقاح "أسترا زينيكا -أوكسفورد " المضاد لكورونا". elbilad.net (in Arabic). Retrieved 1 April 2021.
- "Informació en relació amb la vacunació contra la COVID-19" (PDF) (in Catalan). Govern d'Andorra. Retrieved 14 March 2021.
- AfricaNews (3 March 2021). "Angola begins Covid immunization fight with COVAX vaccines". Africanews. Event occurs at CET13:13:37+01:00. Retrieved 14 April 2021.
- "Argentine regulator approves AstraZeneca/Oxford COVID-19 vaccine". Reuters. 30 December 2020. Retrieved 30 December 2020.
- Ghalechian N (27 April 2021). "Armenia Scraps Restrictions For AstraZeneca Vaccine". «Ազատ Եվրոպա/Ազատություն» ռադիոկայան (in Armenian).
- "COVID Data Tracker". U.S. Centers for Disease Control and Prevention (CDC). 28 March 2020.
- "Bahrain approves Oxford/AstraZeneca coronavirus vaccine produced in India". Saudigazette. 25 January 2021. Retrieved 30 January 2021.
- "Oxford University-Astrazeneca vaccine: Bangladesh okays it for emergency use". The Daily Star. 4 January 2021. Retrieved 6 January 2021.
- "Bangladesh approves Oxford-AstraZeneca COVID-19 vaccine". aa.com.tr. Retrieved 6 January 2021.
- "Bhutan receives 400,000 doses of COVID-19 vaccine from India". Xinhuanet. Retrieved 1 April 2021.
- "Bhutan begins biggest vaccination drive against COVID-19". CNA. Retrieved 1 April 2021.
- "Pfizer/BioNTech COVID-19 vaccines shipped by COVAX arrive in Bosnia and Herzegovina". United Nations. Retrieved 14 April 2021.
- "Botswana starts COVID-19 vaccine rollout". Xinhuanet. Retrieved 1 April 2021.
- "COVID-19: Brunei to begin mass vaccination on April 3". TheScoop. 2 April 2021. Retrieved 6 April 2021.
- "COVID-19 vaccines sent by COVAX arrive in Cabo Verde". ReliefWeb. 15 March 2021. Retrieved 17 April 2021.
- "Instituto de Salud Pública de Chile" (in Spanish). Retrieved 1 April 2021.
- "Colombia approves emergency use of AstraZeneca coronavirus vaccine". Reuters. 23 February 2021. Retrieved 22 March 2021.
- "More than 1.7 million COVID-19 vaccines arrive in the Democratic Republic of Congo". www.unicef.org. Retrieved 25 April 2021.
- "Costa Rica approves use of AstraZeneca's COVID-19 vaccine". Q COSTA RICA. 9 April 2021.
- "La República Dominicana aprueba la vacuna de AstraZeneca contra la covid-19". Agencia EFE (in Spanish). 31 December 2020.
- "First vaccines for East Timor arrive in Dili on Monday – LUSA". COVID-19 Timor-Leste Dashboard. 1 April 2021. Archived from the original on 2 April 2021.
- "Ecuador approves use of AstraZeneca vaccine for COVID-19". Reuters. 24 January 2021. Retrieved 30 January 2021.
- "COVID-19: Egypt authorises Sputnik V, AstraZeneca virus jabs". Gulf News. Retrieved 24 February 2021.
- "El Salvador greenlights AstraZeneca, Oxford University COVID-19 vaccine". Reuters. 30 December 2020.
- "Eswatini launches nationwide COVID-19 vaccination campaign". World Health Organization (WHO). 24 March 2021. Retrieved 1 April 2021.
- "Ethiopia begins COVID-19 vaccine rollout". aa.com.tr. Retrieved 19 March 2021.
- "Ethiopia introduces COVID-19 vaccine in a national launching ceremony". World Health Organization (WHO). Retrieved 19 March 2021.
- "Ethiopia says it has secured 9 million doses of COVID-19 vaccines till April". Reuters. 9 February 2021. Retrieved 19 March 2021.
- "The Arrival of COVAX vaccines Raises Hope in The Gambia". World Health Organization (WHO). Retrieved 19 March 2021.
- "Georgia to receive first doses of COVID-19 vaccine in early March instead of February". Agenda.ge. Retrieved 22 March 2021.
- "Welcome to Ghana Food And Drug Authority". fdaghana.gov.gh. Retrieved 1 April 2021.
- "COVID-19 Information". U.S. Embassy in Guatemala. 25 February 2020. Archived from the original on 7 May 2021. Retrieved 14 April 2021.
- "Today, 28,800 doses of COVID-19 vaccine arrive in Bissau". www.unicef.org.
- "BPOM Terbitkan Izin Penggunaan Darurat Vaksin Covid-19 AstraZeneca". Kompas. Retrieved 10 February 2021.
- "Iran issues permit for emergency use for three other COVID-19 vaccines: Official". IRNA English. 17 February 2021. Retrieved 1 April 2021.
- "Iraq approves Sinopharm, AstraZeneca vaccines". Big News Network. Retrieved 30 January 2021.
- "Covax: Ivory Coast and Ghana begin mass Covid vaccination rollouts". BBC News. 1 March 2021. Retrieved 19 March 2021.
- "Japan Approves Moderna, AstraZeneca Coronavirus Vaccines". Bloomberg. 20 May 2021. Retrieved 2 July 2021.
- "COVAX roll-out - Jordan". www.gavi.org. Retrieved 14 May 2021.
- "Kenya Receives 1M Vaccine Doses, Will Distribute to Health Workers First". Voice of America. 3 March 2021. Retrieved 19 March 2021.
- "Kosovo receives first COVID-19 vaccines through Covax scheme". euronews. 29 March 2021. Retrieved 14 April 2021.
- "Kosovo PM Becomes Nation's First Person To Receive COVID-19 Vaccine". Radio Free Europe/Radio Liberty. 29 March 2021. Retrieved 14 April 2021.
- Godinho V (31 January 2021). "Kuwait authorises emergency use of Oxford-AstraZeneca Covid-19 vaccine". Gulf Business. Retrieved 1 April 2021.
- "Lebanon Received 33600 Doses of The AstraZeneca Vaccine as First Batch". Ministry of Public Health of Lebanon.
- "Lesotho receives 1st batch of COVID-19 vaccines". aa.com.tr. Retrieved 19 March 2021.
- "96,000 Doses of COVID-19 Vaccine Arrives in Liberia". World Health Organization (WHO). Retrieved 11 April 2021.
- "Libya kicks off delayed COVID-19 vaccination drive". Al Jazeera. Retrieved 25 April 2021.
- "57,600 COVID-19 vaccine doses received today in Tripoli, Libya". ReliefWeb. 8 April 2021. Retrieved 25 April 2021.
- "Malawi to receive COVID-19 vaccines in late February – Xinhua". Xinhuanet. Retrieved 19 March 2021.
- "Malawi Sticks to AstraZeneca Despite Concerns Over Efficacy". Voice of America. 8 February 2021. Retrieved 19 March 2021.
- Sipalan J, Donovan K (3 March 2021). "Malaysia approves Sinovac, AstraZeneca COVID-19 vaccines for use". Reuters. Retrieved 7 March 2021.
- "Maldives starts training healthcare workers on COVID-19 vaccine distribution – Xinhua". Xinhuanet. Retrieved 19 February 2021.
- AfricaNews (6 March 2021). "Mali receives first batch of COVID-19 vaccines". Africanews. Retrieved 1 April 2021.
- AfricaNews (26 January 2021). "Mauritius begins vaccinating frontline health workers against covid-19". Africanews. Retrieved 24 February 2021.
- "Mexico approves AstraZeneca COVID-19 vaccine, minister says". Reuters. 5 January 2021. Retrieved 14 January 2021.
- "Moldova approves Russia's Sputnik V vaccine as political row simmers". Thomson Reuters Foundation. Archived from the original on 3 April 2021. Retrieved 1 April 2021.
- "Mongolia approved three Covid-19 vaccines". News.MN. 11 January 2021. Retrieved 1 April 2021.
- "Morocco approves AstraZeneca/Oxford COVID-19 vaccine – Minister". Reuters. 7 January 2021. Retrieved 22 March 2021.
- "Myanmar launches nationwide COVID-19 vaccination program". Xinhua News. 27 January 2021.
- "Importation of COVID-19 Vaccines into Namibia for Public Use and Guidance on Administration of Vaccines by Private Health Care Providers". Republic of Namibia Ministry of Health and Social Services. 31 March 2021. Archived from the original on 22 April 2021. Retrieved 14 April 2021. Public Notice No.: 05/032021
- "Samoa receives 24,000 doses of COVID-19 vaccines through the COVAX facility - Samoa". ReliefWeb. 14 April 2021. Retrieved 7 June 2021.
- "Nepal approves AstraZeneca COVID vaccine for emergency use – government statement". Reuters. 15 January 2021.
- "Wetin we sabi about NAFDAC approval of Oxford AstraZeneca vaccine use for Nigeria". BBC News Pidgin. Retrieved 24 February 2021.
- "Сѐ за вакцините". Официјална веб-страница за вакцинација против КОВИД19 (in Macedonian).
- "Pakistan approves AstraZeneca COVID-19 vaccine for emergency use". 16 January 2021.
- "PNG prime minister first to be vaccinated with Australian-supplied doses 'to show it's safe'". The Guardian. 30 March 2021. Retrieved 1 April 2021.
- "Australia gives Covid-19 vaccine doses to hard-hit Papua New Guinea". France 24. 17 March 2021. Retrieved 1 April 2021.
- "DIGEMID". www.digemid.minsa.gob.pe (in Spanish). Archived from the original on 7 May 2021. Retrieved 2 May 2021.
- "Philippine regulator approves emergency use of AstraZeneca vaccine". Reuters. 28 January 2021. Retrieved 28 January 2021.
- "Rwanda receives COVID-19 Vaccines through COVAX". World Health Organization (WHO). Retrieved 19 March 2021.
- "AstraZeneca and Moderna vaccines to be administered in Saudi Arabia". Gulf News. 18 January 2021. Retrieved 19 January 2021.
- "Serbia receives first shipment of AstraZeneca COVID-19 vaccine". Reuters. 21 February 2021. Retrieved 19 March 2021.
- Bhattacherjee K (22 January 2021). "Coronavirus | Myanmar, Mauritius, Seychelles receive Covishield vaccine". The Hindu. Retrieved 1 April 2021.
- "COVID-19 vaccines shipped by COVAX arrive in Sierra Leone". World Health Organization (WHO). Retrieved 19 March 2021.
- "Those who opt for Sinovac, other vaccines under WHO emergency list to be considered fully vaccinated". CNA. 6 August 2021. Retrieved 6 August 2021.
- "Somalia Receives Its First Batch of COVID-19 Vaccines". Voice of America. 15 March 2021. Retrieved 1 April 2021.
- Kim Hj (10 February 2021). "S. Korea approves AstraZeneca's COVID-19 vaccine for all adults". Yonhap News. Retrieved 10 February 2021.
- Maresca T (10 February 2021). "South Korea approves AstraZeneca COVID-19 vaccine". United Press International. Retrieved 10 February 2021.
- "COVID-19 vaccination kicks-off in South Sudan". World Health Organization (WHO). Retrieved 14 April 2021.
- "Sri Lanka approves vaccine amid warnings of virus spread". Associated Press. 22 January 2021. Retrieved 22 January 2021.
- "COVID-19 vaccination in Sudan". UNICEF. Retrieved 19 March 2021.
- "Sudan receives first delivery of COVID-19 vaccines with over 800,000 doses". UNICEF. Retrieved 19 March 2021.
- "Taiwan grants emergency authorisation for AstraZeneca COVID-19 vaccine". Reuters. 20 February 2021. Retrieved 13 March 2021.
- "Tajikistan becomes first country in Central Asia to receive COVID-19 vaccine through COVAX Facility". unicef.org. Retrieved 14 April 2021.
- "Thai Food and Drug registers COVID-19 vaccine developed by AstraZeneca". Pattaya Mail. 23 January 2021.
- First T. "Covid-19: First vaccination campaign to soon begin". togofirst. Retrieved 19 March 2021.
- "Over 500 people given first COVID-19 vaccine shot in Tonga – Xinhua". xinhuanet. Retrieved 25 April 2021.
- "Tonga's Princess takes the lead on Covid-19 vaccination". RNZ. 16 April 2021. Retrieved 25 April 2021.
- "Kingdom of Tonga receives 24,000 doses of COVID-19 vaccines through the COVAX facility". World Health Organization (WHO). Retrieved 25 April 2021.
- AfricaNews (10 March 2021). "Uganda starts COVID-19 vaccinations". Africanews. Retrieved 19 March 2021.
- "COVID-19: AstraZeneca vaccine certified for use in Ukraine". covid.unian.info. Retrieved 10 March 2021.
- "Information for Healthcare Professionals on COVID-19 Vaccine AstraZeneca". Medicines and Healthcare products Regulatory Agency (MHRA). 30 December 2020. Retrieved 4 January 2021.
- "Oxford University/AstraZeneca vaccine authorised by UK medicines regulator" (Press release). Department of Health and Social Care. 30 December 2020. Retrieved 30 December 2020.
- "Conditions of Authorisation for COVID-19 Vaccine AstraZeneca". Medicines and Healthcare products Regulatory Agency (MHRA). 30 December 2020. Retrieved 4 January 2021.
- "Vietnam approves AstraZeneca COVID-19 vaccine, cuts short Communist Party congress". CNA. Retrieved 5 February 2021.
- "Yemen receives 360,000 COVID-19 vaccine doses through the COVAX Facility". www.unicef.org. Retrieved 7 June 2021.
- "Zambia sets out plans to vaccinate all people over 18 against COVID-19". Reuters. 25 March 2021. Retrieved 17 April 2021.
- "Zambia launches the COVID-19 vaccination". ReliefWeb. 16 April 2021. Retrieved 17 April 2021.
- "Zambia gets 1st 228,000 vaccine doses from COVAX". aa.com.tr. Retrieved 17 April 2021.
- "CARPHA Issues its First Emergency Use Recommendation for a COVID-19 Vaccine". Caribbean Public Health Agency.
- "Suriname begins coronavirus vaccination campaign with donated doses". Reuters. 23 February 2021. Retrieved 28 February 2021.
- Zimmer C, Corum J, Wee SL (10 June 2020). "Coronavirus Vaccine Tracker". The New York Times.
- Psyllides G (31 March 2021). "Coronavirus: 10,000 vaccines handed over to north". cyprus-mail.com/. Retrieved 17 April 2021.
- "COVID-19 Vaccines with WHO Emergency Use Listing". World Health Organization (WHO). 3 November 2021. Archived from the original on 21 December 2021. Retrieved 30 October 2022.
- "AstraZeneca/Oxford Covid-19 Vaccine Gets Emergency Approval From WHO". Forbes. Retrieved 16 February 2021.
- "WHO Recommendation AstraZeneca/SKBio - COVID-19 Vaccine (ChAdOx1-S [recombinant])". World Health Organization (WHO). 15 February 2021. Archived from the original on 4 July 2021. Retrieved 8 May 2021.
- "WHO recommendation Serum Institute of India Pvt Ltd - COVID-19 Vaccine (ChAdOx1-S [recombinant]) - Covishield". World Health Organization (WHO). 15 February 2021. Archived from the original on 6 July 2021. Retrieved 8 May 2021.
- "List of COVID-19 Vaccines Recognised for Specified Purposes" (PDF). Hong Kong Department of Health. Retrieved 24 November 2021.
- FOPH FO. "Coronavirus: Entering Switzerland". www.bag.admin.ch. Archived from the original on 24 July 2020. Retrieved 19 November 2021.
- "COVID-19 and Your Health". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020. Retrieved 17 November 2021.
- "Vaxzevria". Union Register of medicinal products. Retrieved 26 November 2021.
- "Regulatory Decision Summary – Pfizer-BioNTech COVID-19 Vaccine". Health Canada, Government of Canada. 9 December 2020. Retrieved 9 December 2020.
- "Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Adults". ClinicalTrials.gov. United States National Library of Medicine. 30 April 2020. NCT04368728. Archived from the original on 11 October 2020. Retrieved 14 July 2020.
- "A Multi-site Phase I/II, 2-Part, Dose-Escalation Trial Investigating the Safety and Immunogenicity of four Prophylactic SARS-CoV-2 RNA Vaccines Against COVID-19 Using Different Dosing Regimens in Healthy Adults". EU Clinical Trials Register. European Union. 14 April 2020. EudraCT 2020-001038-36. Archived from the original on 22 April 2020. Retrieved 22 April 2020.
- Burger L (16 March 2020). "BioNTech in China alliance with Fosun over potential coronavirus vaccine". Reuters. Retrieved 18 April 2021.
- "BioNTech, Fosun start Phase II trial of COVID-19 vaccine in China". Reuters. 25 November 2020. Retrieved 18 April 2021.
- Kemp R (11 July 2021). "Fosun says it has struck deal to send Taiwan vaccines". RTHK. Retrieved 11 July 2021.
- "10 Million Doses of mRNA-based COVID-19 Vaccine to be supplied to Taiwan Region". BioNTech (Press release). 12 July 2021. Retrieved 18 July 2021.
- "Comirnaty". Therapeutic Goods Administration (TGA). 25 January 2021. Retrieved 25 January 2021.
- Australian Public Assessment Report for BNT162b2 (mRNA) (PDF) (Report). Therapeutic Goods Administration (TGA). Retrieved 25 January 2021.
- "Vacina da Pfizer é a 1ª a obter registro definitivo no Brasil" (in Brazilian Portuguese). G1. 23 February 2021. Retrieved 23 February 2021.
- "Pfizer-BioNTech Comirnaty COVID-19 vaccine". Health Canada. 21 October 2022. Retrieved 30 October 2022.
- Osman L (16 September 2021). "Goodbye Pfizer, hello Comirnaty: Top COVID-19 vaccines given brand names in Canada". CBC News. Retrieved 16 September 2021.
- "Robust assessment ahead of Medsafe approval of vaccine". Ministry of Health. 3 February 2021. Retrieved 6 February 2021.
- "Interior Applauds Inclusion of Insular Areas through Operation Warp Speed to Receive COVID-19 Vaccines" (Press release). United States Department of the Interior (DOI). 12 December 2020. Retrieved 13 January 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Dorman B (6 January 2021). "Asia Minute: Palau Administers Vaccines to Keep Country Free of COVID". Hawaii Public Radio. Retrieved 13 January 2021.
- "Coronavirus: Saudi Arabia approves Pfizer COVID-19 vaccine for use". Al Arabiya English. 10 December 2020. Retrieved 10 December 2020.
- "Swissmedic grants authorisation for the first COVID-19 vaccine in Switzerland" (Press release). Swiss Agency for Therapeutic Products (Swissmedic). 19 December 2020. Retrieved 19 December 2020.
- "Comirnaty Product information" (in German). Retrieved 31 January 2021. [Due to incomplete clinical data at the time of the assessment of the authorization application, the Comirnaty medicinal product is authorized for a limited period (Art. 9a Medicinal Products Act).]
- "Use of vaccine approved". Government of Hong Kong. 25 January 2021. Retrieved 15 February 2021.
- "COVID-19 vaccines supplied by two drug manufacturers registered as pharmaceutical products in Hong Kong". Government of Hong Kong. 20 December 2022. Retrieved 23 December 2022.
- "Canada allows Pfizer COVID-19 vaccine for children aged 12-15". Reuters. 5 May 2021. Retrieved 19 August 2021.
- "Andorra COVID-19". govern.ad. Retrieved 19 February 2021.
- "Coronavirus en la Argentina: La ANMAT aprobo el uso de emergencia de la vacuna Pfizer". La Nación (in Spanish). Retrieved 23 December 2020.
- "Dashboard: Vaccinating Eurasia- June". Eurasianet. 30 June 2021. Retrieved 4 August 2021.
- "Bahrain becomes second country to approve Pfizer COVID-19 vaccine". Al Jazeera. Retrieved 5 December 2020.
- "Bangladesh approves Pfizer Covid-19 vaccine for emergency use". Dhaka Tribune. 27 May 2021. Retrieved 27 May 2021.
- "Botswana expects to welcome multiple COVID-19 vaccines". Xinhuanet. Retrieved 1 April 2021.
- "Chile approves Pfizer-BioNTech Covid-19 vaccine for emergency use". The Straits Times. 17 December 2020. Retrieved 17 December 2020.
- "BioNTech ships COVID shots to China for use by Germans". Reuters. 22 December 2022. Retrieved 23 December 2022.
- "Colombia regulator approves Pfizer-BioNTech vaccine for emergency use". Reuters. 6 January 2021. Retrieved 6 January 2021.
- "Covid-19 en RDC: des officiers et agents de la Police se font vacciner après échanges avec le ministre de la Santé". Actualite.cd (in French). 7 December 2021. Retrieved 12 January 2022.
- "Costa Rica authorizes Pfizer-BioNTech coronavirus vaccine". The Tico Times. 16 December 2020. Retrieved 16 December 2020.
- Pfizer-BioNTech COVID-19 vaccine. Pan American Health Organization.
- "Arcsa autoriza ingreso al país de vacuna Pfizer-BioNTech para el Covid-19 – Agencia Nacional de Regulación, Control y Vigilancia Sanitaria" (in Spanish). Retrieved 17 December 2020.
- McGwin K (30 December 2020). "Greenland to begin new year with COVID vaccinations". ArcticToday. Retrieved 21 March 2021.
- "Vaccine FAQs – MINISTRY OF HEALTH & MEDICAL SERVICES". 24 March 2021. Retrieved 6 January 2022.
- "Ghana approves two more COVID-19 vaccines - Xinhua | English.news.cn". xinhuanet. Retrieved 30 November 2021.
- "BPOM Terbitkan Izin Penggunaan Darurat Vaksin COVID-19 Pfizer". CNN Indonesia (in Indonesian). 15 July 2021. Retrieved 15 July 2021.
- "Iraq grants emergency approval for Pfizer COVID-19 vaccine". MSN. Retrieved 27 December 2020.
- "Israeli Health Minister 'pleased' as FDA approves Pfizer COVID-19 vaccine". The Jerusalem Post. 12 December 2020. Retrieved 28 December 2020.
- "Japan approves Pfizer's COVID-19 vaccine, 1st for domestic use". Nikkei Asia. 14 February 2021. Archived from the original on 18 March 2021. Retrieved 14 February 2021.
- Tsukimori O (15 February 2021). "Japan gives OK to Pfizer coronavirus vaccine, with rollout to begin this week". The Japan Times. Retrieved 1 April 2021.
- "Jordan approves Pfizer-BioNTech Covid vaccine". France 24. 15 December 2020. Retrieved 15 December 2020.
- "Kenya Approves J&J, Pfizer Covid-19 Vaccines as Cases Surge". Bloomberg.com. 2 August 2021. Retrieved 24 September 2021.
- "Kuwait authorizes emergency use of Pfizer-BioNTech COVID-19 vaccine". Arab News. 13 December 2020. Retrieved 15 December 2020.
- "Lebanon starts Covid-19 vaccination campaign". France 24. 14 February 2021. Retrieved 16 March 2021.
- "Malawi Ministry of Health on Facebook". Facebook. Retrieved 14 September 2021.
- Lim I (8 January 2021). "Khairy: Malaysia can use Pfizer's Covid-19 vaccine now as conditional registration granted". The Malay Mail. Retrieved 8 January 2021.
- "Sinopharm & Pfizer approved for use in Maldives". The Times of Addu. 15 March 2021. Retrieved 1 April 2021.
- "Mexico Approves Pfizer Vaccine for Emergency Use as Covid Surges". Bloomberg. 12 December 2020. Retrieved 12 December 2020.
- "Mexico authorizes emergency use of Pfizer-BioNTech coronavirus vaccine". The Economic Times. 12 December 2020. Retrieved 14 January 2021.
- "Mongolia will receive 300,000 doses of COVID-19 vaccine developed by China". AKIpress News Agency. Retrieved 19 March 2021.
- "People to get Pfizer vaccine from today". himalayantimes. 14 November 2021. Retrieved 28 December 2021.
- "NAFDAC approves Pfizer Biotech Vaccine for emergency use authorisation". The Guardian Nigeria News - Nigeria and World News. 30 April 2021. Retrieved 21 July 2021.
- "North Macedonia Kicks Off COVID-19 Vaccinations As Serbia Looks To Highlight Regional Efforts". Radio Free Europe/Radio Liberty. 18 February 2021. Retrieved 19 February 2021.
- "Oman issues licence to import Pfizer BioNTech Covid vaccine – TV". Reuters. 15 December 2020. Retrieved 16 December 2020.
- "Panama approves Pfizer's COVID-19 vaccine – health ministry". Yahoo! Finance. 16 December 2020. Retrieved 16 December 2020.
- "Vaccination is not compulsory, Prime Minister tells Parliament". PNG | COVID 19. 12 August 2021. Retrieved 24 September 2021.
- "Peru regulators approve Pfizer vaccine against COVID-19: filing". Yahoo! News. 2 February 2021. Retrieved 31 March 2021.
- "PH authorizes Pfizer's COVID-19 vaccine for emergency use". CNN Philippines. 14 January 2021. Archived from the original on 14 January 2021. Retrieved 14 January 2021.
- "Qatar, Oman to receive Pfizer-BioNTech COVID-19 vaccine this week". Reuters. Retrieved 24 December 2020.
- "Serbia leads region in expecting COVID-19 vaccines within days". BalkanInsight. 21 December 2020. Retrieved 26 December 2020.
- "First shipment of Pfizer-BioNTech vaccine arrives in Serbia". Serbian government website. 22 December 2020. Retrieved 26 December 2020.
- "Singapore approves use of Pfizer's COVID-19 vaccine". Associated Press. 14 December 2020. Retrieved 15 December 2020.
- "South Africa approves Pfizer Covid-19 vaccine for emergency use". Retrieved 18 March 2021.
- "Pfizer-BioNTec approved for emergency use in SA: here is what you need to know about the vaccine". TimesLIVE. Retrieved 19 March 2021.
- Lee Ke (27 February 2021). "S. Korea begins rolling out Pfizer vaccines on second day of national vaccination program". Arirang News. Archived from the original on 10 March 2021. Retrieved 28 February 2021.
- Kim Hj (5 March 2021). "S. Korea approves Pfizer's COVID-19 vaccine amid immunization push". Yonhap News. Retrieved 5 March 2021.
- "Sri Lanka approves Pfizer COVID vaccine for emergency use". Reuters. 8 May 2021.
- "Thailand approves Pfizer-Biontech vaccine for emergency use". Reuters. 24 June 2021.
- Gözde Bayar (18 March 2021). "Turkey to receive 4.5M doses of Pfizer vaccine this month". Anadolu Agency.
- "Dubai approves the Pfizer-BioNTech vaccine which will be free of charge". Emirates Woman. 23 December 2020. Retrieved 28 December 2020.
- "UK medicines regulator gives approval for first UK COVID-19 vaccine". Medicines and Healthcare Products Regulatory Agency, Government of the UK. 2 December 2020. Retrieved 2 December 2020.
- "Information for Healthcare Professionals on Pfizer/BioNTech COVID-19 vaccine". Medicines & Healthcare products Regulatory Agency (MHRA). 8 December 2020. Retrieved 13 December 2020.
- "Conditions of Authorisation for Pfizer/BioNTech COVID-19 vaccine". Medicines & Healthcare products Regulatory Agency (MHRA). 3 December 2020. Retrieved 13 December 2020.
- "U.S. Donates Over 1.2 million COVID-19 Pfizer Vaccines to Uzbekistan". [[U.S. Embassy in Uzbekistan. 12 September 2021. Retrieved 4 October 2021.
- McElwee JJ (11 December 2020). "Vatican to offer coronavirus vaccine to residents and staff in early 2021". National Catholic Reporter. Retrieved 9 February 2021.
- Menichetti M (11 December 2020). "In Vaticano parte il piano vaccinale anticovid". vatican.va (Press release). Retrieved 9 February 2021.
- Le N (12 June 2021). "Vietnam approves Pfizer Covid vaccine for emergency use". VnExpress.
- "COVID19 Vaccine Tracker". covid19.trackvaccines.org. Retrieved 12 January 2022.
- "Caribbean Public Health Agency > Who We Are > Member States". The Caribbean Public Health Agency (CARPHA). Retrieved 7 April 2021.
- "Pfizer vaccine approved for region but Health Ministry wants more info". Trinidad and Tobago Newsday. 6 January 2021. Retrieved 24 February 2021.
- Nielsdóttir A (30 December 2020). "First Covid-19 vaccines administered in the Faroe Islands". Local.fo. Retrieved 29 March 2021.
- Sevunts L, International RC (4 January 2021). "Greenland launches COVID-19 vaccination campaign". Eye on the Arctic. Retrieved 21 March 2021.
- "Local BioNTech vaccination to resume on April 5". macaubusiness.com. 2 April 2021. Retrieved 15 April 2021.
- "WHO issues its first emergency use validation for a COVID-19 vaccine and emphasizes need for equitable global access". World Health Organization (WHO) (Press release). 31 December 2020. Retrieved 6 January 2021.
- "WHO recommendation Tozinameran – COVID-19 mRNA vaccine (nucleoside modified) – Comirnaty". World Health Organization (WHO). 31 December 2020. Archived from the original on 9 May 2021. Retrieved 8 May 2021.
- "Comirnaty". European Medicines Agency (EMA). 13 September 2022.
- "Comirnaty bivalent jab use authorised". news.gov.hk. 18 November 2022. Retrieved 18 November 2022.
- "Comirnaty Bivalent Vaccine Arrives in Hong Kong SAR and Macau SAR and Will Soon Be Available to the Local Residents". Fosun Pharma. 28 November 2022. Retrieved 6 January 2023.
- "Pfizer-BioNTech COVID-19 Vaccine". U.S. Food & Drug Administration. 11 December 2023. Retrieved 26 January 2024.
- "Janssen COVID-19 Emergency Use Authorization (EUA) Official Website". Janssen. 28 February 2021. Retrieved 28 February 2021.
- Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19 (Briefing). U.S. Food and Drug Administration (FDA). 26 February 2021. p. 6. Retrieved 6 March 2021.
The vaccine, known as Ad26.COV2.S, is a replication-incompetent adenovirus type 26 (Ad26) vectored vaccine encoding a stabilized variant of the SARS-CoV-2 S protein.
- "A Study of Ad26.COV2.S in Adults". ClinicalTrials.gov. 4 August 2020. Archived from the original on 16 September 2020. Retrieved 23 August 2020.
- "A Study of Ad26.COV2.S for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adult Participants". ClinicalTrials.gov. US National Library of Medicine. Archived from the original on 26 September 2020.
- "COVID-19 Vaccine Janssen EPAR". European Medicines Agency (EMA). 5 March 2021. Retrieved 16 March 2021.
- "Decision on using the Johnson & Johnson coronavirus vaccine in Finland to come after European Medicines Agency issues recommendations - News - THL". Finnish Institute for Health and Welfare (THL), Finland. Retrieved 5 December 2021.
- "FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine" (Press release). U.S. Food and Drug Administration (FDA). 27 February 2021. Retrieved 27 February 2021.
- "(CDC) Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Recommendations from the Advisory Committee on Immunization Practices — United States, December 2021". U.S. Food & Drug Administration. 21 January 2022.
- "(CDC) Janssen (Johnson & Johnson) COVID-19 Vaccine". U.S. Food & Drug Administration. 10 May 2023.
- "COVID-19 Vaccine Janssen". Therapeutic Goods Administration. 25 June 2021. Retrieved 26 June 2021.
- "TGA grants third provisional approval to COVID-19 vaccine: Janssen". Therapeutic Goods Administration (TGA). 25 June 2021. Retrieved 28 June 2021.
- "Janssen Jcovden (Johnson & Johnson) COVID-19 vaccine". Health Canada. 21 September 2022. Retrieved 30 October 2022.
- "Regulatory Decision Summary - Janssen COVID-19 Vaccine". Health Canada. Retrieved 24 November 2021.
- "Johnson & Johnson Covid vaccine approved for use in Switzerland". SWI swissinfo.ch. Keystone-SDA/Reuters/sb. Retrieved 1 April 2021.
- "Swissmedic regulator approves Johnson & Johnson COVID-19 vaccine". Reuters. 22 March 2021. Retrieved 3 April 2021.
- "COVID-19: la ANMAT aprobó el uso de emergencia de la vacuna de Johnson & Johnson". infobae (in European Spanish). 6 October 2021. Retrieved 6 October 2021.
- "Farmacovigilancia de vacunas para COVID-19 - Janssen". Farmacovigilancia de vacunas para COVID-19. Retrieved 9 October 2021.
- "Bahrain first to approve Johnson & Johnson COVID-19 vaccine for emergency use". Reuters. 25 February 2021. Retrieved 25 February 2021.
- "Bahrain becomes 1st nation to grant J&J shot emergency use". ABC News (United States). 25 February 2021. Retrieved 25 February 2021.
- "Bangladesh approves Johnson & Johnson's Covid-19 vaccine for emergency use". Dhaka Tribune. 15 June 2021. Retrieved 15 June 2021.
- "Single-dose Covid-19 vaccine Janssen gets emergency use authorization in Bangladesh". The Daily Star. 15 June 2021. Retrieved 15 June 2021.
- "COVID-19 Vaccines Approved by BoMRA". www.bomra.co.bw. Archived from the original on 13 June 2021. Retrieved 11 June 2021.
- "Brazil health regulator approves emergency use of Johnson & Johnson COVID-19 vaccine". Rio de Janeiro: Reuters. 31 March 2021. Retrieved 1 April 2021.
- "Chile authorizes emergency use of Johnson & Johnson's Janssen vaccine through COVAX". Public Health Institute, Ministry of Health of Chile. 10 June 2021.
- Acosta LJ (25 March 2021). "Colombia grants emergency use for J&J coronavirus vaccine". Bogotá: Reuters. Retrieved 25 March 2021.
- "India approves J&J COVID-19 vaccine for emergency use". Reuters. 7 August 2021. Retrieved 7 August 2021.
- "BPOM Terbitkan Izin Penggunaan Darurat Vaksin Covid-19 Produksi Johnson & Johnson dan Cansino". KOMPAS.com (in Indonesian). 7 September 2021. Retrieved 7 September 2021.
- "Kuwait authorises emergency use of Johnson & Johnson vaccine". Kuwait News Agency (KUNA). 8 June 2021.
- "Malaysia grants conditional approval for CanSino, J&J COVID-19 vaccines". Reuters. 15 June 2021. Retrieved 15 June 2021.
- "Approval to use of Covid-19 vaccine Janssen" (PDF). Maldives Food and Drug Authority. 6 June 2021. Archived from the original (PDF) on 11 June 2021. Retrieved 11 June 2021.
- "COFEPRIS emite autorización para uso de emergencia a vacuna contra COVID-19 Ad26.COV2.S de Janssen (Johnson & Johnson)". www.gob.mx/cofepris (in Spanish).
- "Membrii NITAG au venit cu recomandări privind utilizarea vaccinurilor împotriva COVID-19 în Republica Moldova". Ministerul Sănătății, Muncii și Protecţiei Sociale (in Romanian). 3 March 2021.
- "1.5 million doses of J&J vaccine arrive in Nepal". kathmandupost. Retrieved 12 July 2021.
- "905m COVID-19 shots administered worldwide – Report". 20 April 2021. Retrieved 7 June 2021.
- "Peru authorizes use of Johnson & Johnson COVID-19 vaccine". Andina. 9 July 2021. Retrieved 3 August 2021.
- "Covaxin, Janssen approved for emergency use in PH". CNN Philippines. 19 April 2021. Archived from the original on 19 April 2021.
- "Coronavirus: South Africa rolls out vaccination programme". BBC News. 17 February 2021. Retrieved 19 February 2021.
- Lardieri A (7 April 2021). "South Korea Approves Johnson & Johnson Vaccine". U.S. News & World Report. Retrieved 8 April 2021.
- "嬌生疫苗通過我緊急使用授權 BNT僅「有條件通過」". The Liberty Times. 10 June 2021.
- "Thailand approves Johnson & Johnson's COVID-19 vaccine". Reuters. 25 March 2021. Retrieved 1 April 2021.
- "Tunisia approve Johnson & Johnson COVID-19 vaccine". Reuters. 8 April 2021.
- "Janssen single-dose Covid vaccine approved by UK". BBC News. 28 May 2021.
- "Janssen COVID-19 Vaccine EUA Letter of Authorization 06102021". U.S. Food & Drug Administration. 10 June 2021.
- "Việt Nam phê duyệt khẩn cấp vaccine COVID-19 của Johnson & Johnson". Lao Động. 15 July 2021.
- "Bộ Y tế phê duyệt vaccine phòng COVID-19 Janssen của Johnson & Johnson". Vietnam+. 15 July 2021.
- "Zimbabwe approves J&J COVID-19 vaccine for emergency use". Reuters. 28 July 2021. Retrieved 23 August 2021.
- "COVID-19 Vaccine Janssen". Union Register of medicinal products. Retrieved 26 November 2021.
- "COVID-19 Vaccine Information". www.carpha.org.
- "Public Health (Emergency Authorisation of COVID-19 Vaccine) Rules, 2021" (PDF). Government of Saint Vincent and the Grenadines. 11 February 2021. Retrieved 12 February 2021.
- "Aşı Türleri - K.K.T.C. Sağlık Bakanlığı - Aşı Bilgi Sistemi". asi.saglik.gov.ct.tr. Retrieved 19 November 2021.
- "UPDATE: Statement to Member States regarding thromboembolic events after vaccination with Janssen COVID-19 vaccine (Johnson & Johnson)". Africa CDC. Retrieved 7 June 2021.
- "WHO approves J&J's COVID-19 vaccine for emergency listing". Channel NewsAsia. 13 March 2021. Archived from the original on 18 March 2021. Retrieved 13 March 2021.
- "WHO recommendation Janssen–Cilag International NV (Belgium) COVID-19 Vaccine (Ad26.COV2-S [recombinant])". World Health Organization (WHO). 12 March 2021. Archived from the original on 8 May 2021. Retrieved 8 May 2021.
- "Requirements for a valid vaccination certificate (3 doses)" (PDF). Quarantine Station, Ministry of Health, Labour and Welfare, the Government of Japan. Archived from the original (PDF) on 5 October 2022. Retrieved 5 October 2022.
- "Vaccinations for Turkey: Do I need an Approved COVID Vaccine?". Turkey Visa. 10 April 2019. Retrieved 24 November 2021.[permanent dead link]
- "Spikevax (previously COVID-19 Vaccine Moderna) EPAR". European Medicines Agency (EMA). 4 January 2021.
- "Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19)". ClinicalTrials.gov. United States National Library of Medicine. 16 March 2020. NCT04283461. Retrieved 5 March 2021.
- "A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19". ClinicalTrials.gov. United States National Library of Medicine. 14 July 2020. NCT04470427. Archived from the original on 11 October 2020. Retrieved 27 July 2020.
- Palca J (27 July 2020). "COVID-19 vaccine candidate heads to widespread testing in U.S." NPR. Archived from the original on 11 October 2020. Retrieved 27 July 2020.
- "THL issues instructions that men under 30 years of age should only be offered the Comirnaty coronavirus vaccine - Press release - THL". Finnish Institute for Health and Welfare (THL), Finland. Retrieved 5 December 2021.
- "TGA provisionally approves Moderna's COVID-19 vaccine". Therapeutic Goods Administration. 9 August 2021.
- "Moderna Spikevax COVID-19 vaccines". Health Canada. 1 September 2022. Retrieved 30 October 2022.
- "Regulatory Decision Summary – Moderna COVID-19 Vaccine". Health Canada, Government of Canada. 23 December 2020. Retrieved 23 December 2020.
- "Drug and vaccine authorizations for COVID-19: List of applications received". Health Canada, Government of Canada. 9 December 2020. Retrieved 9 December 2020.
- "Swissmedic grants authorisation for the COVID-19 vaccine from Moderna" (Press release). Swiss Agency for Therapeutic Products (Swissmedic). 12 January 2020. Retrieved 12 January 2020.
- "Information for Healthcare Professionals on COVID-19 Vaccine Moderna". Medicines and Healthcare products Regulatory Agency (MHRA). 1 April 2021. Retrieved 6 April 2021.
- "Summary of the Public Assessment Report for COVID-19 Vaccine Moderna". Medicines and Healthcare products Regulatory Agency (MHRA). 19 February 2021. Retrieved 6 April 2021.
- "Spikevax". Union Register of medicinal products. Retrieved 26 November 2021.
- "BOLETIN OFICIAL REPUBLICA ARGENTINA - MINISTERIO DE SALUD - Resolución 2711/2021". www.boletinoficial.gob.ar. Retrieved 6 October 2021.
- "Moderna gets emergency use authorisation in Bangladesh". The Daily Star. 29 June 2021. Retrieved 30 June 2021.
- "Moderna Announces Agreement to Supply the Republic of Botswana with its COVID-19 Vaccine" (Press release). Moderna. 3 June 2021 – via Business Wire.
- "Covid-19: aumenta para sete o número de vacinas autorizadas no Brasil". Agência Brasil (in Brazilian Portuguese). 7 May 2021.
- "Instituto de Salud Pública de Chile". www.ispch.cl. Retrieved 21 May 2022.
- Espectador E (25 June 2021). "Colombia autoriza el uso de la vacuna de Moderna para el coronavirus". El Espectador.
- "StackPath". dailynewsegypt. 31 October 2021. Retrieved 5 November 2021.
- "Farmacovigilancia de vacunas para COVID-19 - Moderna". Farmacovigilancia de vacunas para COVID-19. Retrieved 24 September 2021.
- "Honduras a la zaga de la vacunación en Centroamérica". Proceso Digital (in Spanish). 10 April 2021.
- Kaul R (29 June 2021). "Moderna's Covid vaccine approved for use in India". Hindustan Times.
- Friski Riana (2 July 2021). Laila Afifa (ed.). "BPOM Issues Emergency Use Authorization for Moderna Vaccine". Tempo.co.
- "Israeli Ministry of Health Authorizes COVID-19 Vaccine Moderna for Use in Israel". modernatx.com. 4 January 2021. Archived from the original on 17 February 2021. Retrieved 4 January 2021.
- "Takeda Announces Approval of Moderna's COVID-19 Vaccine in Japan" (Press release). Takeda. 21 May 2021. Retrieved 2 July 2021 – via Business Wire.
- "Kenya approves Moderna Sinopharm vaccines amid fourth wave". Bloomberg.com. 19 August 2021. Retrieved 28 August 2021.
- "COVID-19: Kuwait unlikely to import Sputnik V". gulfnews.com. 7 April 2021. Retrieved 21 April 2021.
- New Straits Times (8 August 2021). "DCA grants conditional approval for Moderna vaccine".
- Maldives Food and Drug Agency (6 June 2021). "Approval to use of COVID-19 vaccine Moderna dispersion for injection" (PDF). Archived from the original (PDF) on 6 September 2021. Retrieved 11 June 2021.
- "Comité de Moléculas Nuevas y Subcomité emiten opinión favorable para vacuna Moderna". www.gob.mx/cofepris (in Spanish).
- "COFEPRIS emite autorización para uso de emergencia a vacuna Moderna". www.gob.mx/cofepris (in Spanish).
- "Nepal receives 1,965,600 doses of Moderna vaccine from COVAX". kathmandupost.com. Retrieved 28 December 2021.
- "NAFDAC approves emergency use of three additional Covid-19 vaccines for Nigeria". The Guardian Nigeria News - Nigeria and World News. 15 July 2021. Retrieved 21 July 2021.
- "Gobierno suscribió acuerdo con Moderna por 20 millones de dosis contra la COVID-19". RPP Noticias. 17 September 2021. Retrieved 4 October 2021.
- Galvez, Daphne (5 May 2021). "Moderna COVID-19 vaccine gets emergency use authorization". Philippine Daily Inquirer.
- "Qatar approves emergency use of Moderna's COVID-19 vaccine". Al Jazeera. Retrieved 31 March 2021.
- "Moderna arrived to Serbia". 4 November 2021.
- "Singapore becomes first in Asia to approve Moderna's COVID-19 vaccine". Reuters. 3 February 2021. Retrieved 3 February 2021.
- Kim Hj (21 May 2021). "Moderna COVID-19 vaccine gets final nod in S. Korea". Yonhap News Agency. Retrieved 21 May 2021.
- "莫德納疫苗獲緊急授權 自費AZ疫苗供應不間斷". Central News Agency. 5 May 2021.
- "1.5 million doses of Moderna Vaccines arrive in Tajikistan". UNICEF. 26 July 2021. Retrieved 4 August 2021.
- "Thai FDA approves Moderna vaccine". Bangkok Post. 13 May 2021.
- "U.S. Donates 2 Million Coronavirus Vaccine Doses To Ukraine". RadioFreeEurope. 19 July 2021. Retrieved 4 August 2021.
- "Coronavirus latest: Moderna vaccine approved for emergency use by UAE". The National. 4 July 2021.
- "U.S. Government Provides 3 Million of the Moderna COVID-19 Vaccine to Uzbekistan". 30 July 2021. Retrieved 4 August 2021.
- "Bộ Y tế phê duyệt có điều kiện vaccine COVID-19 Moderna". Vietnam Government Portal. 29 June 2021.
- "WHO lists anti-COVID Moderna vaccine for emergency use". Agence France-Presse. Philippine Daily Inquirer. 1 May 2021.
- "WHO Recommendation COVID-19 mRNA Vaccine (nucleoside modified)". World Health Organization (WHO). 30 April 2021. Retrieved 8 May 2021.
- "Vaccination requirements for travel to New Zealand". Unite against COVID-19. 4 January 2023.
- "TGA provisionally approves Moderna's Omicron booster for Australia". www.9news.com.au. 30 August 2022. Retrieved 5 September 2022.
- Boisvert N (1 September 2022). "Health Canada approves updated Moderna vaccine for Omicron variant". CBC. Retrieved 3 September 2022.
- "New bivalent COVID-19 booster vaccine doses expected to be available in Singapore by end-Sep: Moderna". Channel News Asia. 15 September 2022. Retrieved 24 September 2022.
- "Swissmedic approves first bivalent COVID-19 booster vaccine in Switzerland". Swissmedic. 29 August 2022. Retrieved 5 September 2022.
- "First batch of 804,000 doses of Moderna's next-generation bivalent COVID-19 vaccine to arrive in Taiwan on evening of September 16". Taiwan Centers for Disease Control. 16 September 2022. Retrieved 21 September 2022.
- Shen Py, Shih Hc (2 September 2022). "Taiwan grants EUA for 2nd-generation Moderna COVID-19 vaccine". Focus Taiwan. Retrieved 25 September 2022.
- "Covid: UK first country to approve dual-strain vaccine". BBC News. 16 August 2022. Retrieved 4 September 2022.
- "Spikevax (previously COVID-19 Vaccine Moderna)". European Medicines Agency (EMA). 2 September 2022.
- "First batch of 703,000 doses of Moderna BA.4/5 bivalent COVID-19 vaccine to arrive in Taiwan on morning of November 10". Taiwan Centers for Disease Control. 9 November 2022. Retrieved 15 December 2022.
- "Moderna COVID-19 Vaccine". U.S. Food & Drug Administration. 1 November 2023. Retrieved 26 January 2024.
- Chen W, Al Kaabi N (18 July 2020). "A Phase III clinical trial for inactivated novel coronavirus pneumonia (COVID-19) vaccine (Vero cells)". Chinese Clinical Trial Registry. Archived from the original on 11 October 2020. Retrieved 15 August 2020.
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. (October 2020). "Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial". The Lancet. Infectious Diseases. 21 (1): 39–51. doi:10.1016/s1473-3099(20)30831-8. PMC 7561304. PMID 33069281.
- "Bahrain approves China's Sinopharm coronavirus vaccine". Arabian Business. 13 December 2020.
- Kuo L. "China approves Sinopharm coronavirus vaccine, the country's first for general use". The Washington Post.
- "President Ramkalawan and First Lady receives second dose SinoPharm Vaccine". statehouse.gov.sc. Retrieved 5 February 2021.
- Wee SL (9 December 2020). "Chinese Covid-19 Vaccine Gets Key Push, but Doubts Swirl". The New York Times. Retrieved 12 December 2020.
- "Coronavirus: UAE authorises emergency use of vaccine for frontline workers". The National. 14 September 2020. Retrieved 24 November 2020.
- "China 'to provide 400,000 COVID vaccine doses' to Afghanistan". Al Jazeera. Retrieved 22 March 2021.
- Presse AA. "Algeria Receives 200,000 Coronavirus Jabs From China". Barrons. Retrieved 22 March 2021.
- "Angola recebeu doação chinesa de 200 mil doses de vacinas Sinopharm". Notícias ao Minuto (in Portuguese). 25 March 2021. Retrieved 14 April 2021.
- Biannchi W (21 February 2021). "Argentina approves Sinopharm COVID-19 vaccine for emergency use". Reuters. Retrieved 23 February 2021.
- "Bangladesh approves emergency use of China's Sinopharm vaccine amid supply squeeze". bdnews24.com. 29 April 2021. Retrieved 29 April 2021.
- "Belarus begins COVID-19 vaccinations with Chinese shots". Belta. 15 March 2021. Retrieved 16 March 2021.
- "Bhutan receives 500,000 doses of COVID-19 vaccines through COVAX". www.unicef.org. Retrieved 15 July 2021.
- "Bolivia begins inoculation with Sinopharm jabs". The Star. Malaysia. Retrieved 28 February 2021.
- "Vaccine donation from China arrives". The Star. Malaysia. Retrieved 22 March 2021.
- Rinith T (4 February 2021). "Health Ministry grants Emergency Use Authorization to China's Sinopharm vaccine". Khmer Times. Retrieved 4 February 2021.
- AfricaNews (6 June 2021). "Chadians line up for Sinopharm as COVID-19 vaccination campaign begins". Africanews. Retrieved 6 June 2021.
- "Cuba aplicará vacuna china Sinopharm junto a inoculaciones de desarrollo local". euronews (in Spanish). 28 August 2021. Retrieved 29 August 2021.
- "Ministro de Salud recibe su primera dosis de la vacuna Sinovac; asegura que el biológico es confiable". Ministerio de Salud Pública (in Spanish). 3 May 2021.
- "StackPath". dailynewsegypt. 17 February 2021. Retrieved 22 March 2021.
- "Ethiopia to get 300,000 doses of Sinopharm COVID-19 shot, health minister says". Reuters. 29 March 2021. Retrieved 14 April 2021.
- "Gabon receives 100,000 doses of Sinopharm's vaccine from China". Gabon 24. 12 March 2021. Retrieved 15 March 2021.
- "Georgia Starts COVID-19 Vaccination with Sinopharm". Civil.ge. 4 May 2021. Retrieved 9 May 2021.
- Spike J (22 March 2021). "Hungary approves 2 more vaccines from outside EU amid spike". ABC News. Associated Press. Retrieved 14 July 2021.
- "Indonesia approves Sinopharm COVID-19 vaccine for emergency use". Kontan. 30 April 2021. Retrieved 30 April 2021.
- "Iran Launches Phase Two of Mass Inoculation Campaign". Financial Tribune. 22 February 2021. Retrieved 23 February 2021.
- "First batch of Chinese Sinopharm vaccine arrives in Jordan". Roya News. Retrieved 9 January 2021.
- Satubaldina A (30 April 2021). "Three Vaccines to Become Available to Kazakh Citizens". The Astana Times. Retrieved 1 May 2021.
- Kharizov R (19 March 2021). "150,000 doses of Sinopharm coronavirus vaccine delivered to Kyrgyzstan". 24.kg. Retrieved 22 March 2021.
- "Laos declares Covid-19 vaccinations safe, more to be inoculated next week". The Star. Malaysia. Retrieved 19 February 2021.
- Crean R. "China donates 50,000 doses of Sinopharm vaccine to Lebanon". The Daily Star. Retrieved 2 March 2021.
- "Libya gets 2 million Sinopharm doses with more expected". Africanews. 2 August 2021. Retrieved 5 August 2021.
- Zimmer C, Corum J, Wee SL (10 June 2020). "Coronavirus Vaccine Tracker". The New York Times. ISSN 0362-4331. Retrieved 29 August 2021.
- Latiff R (16 July 2021). "Malaysia approves China's Sinopharm vaccine for emergency use". Reuters. Retrieved 16 July 2021.
- Rasheed AH. "MFDA approves Pfizer, Sinopharm Covid-19 vaccines for emergency use". raajje.mv. Retrieved 16 March 2021.
- "Mauritania begins COVID-19 vaccination campaign". aa.com.tr. Retrieved 29 March 2021.
- "Comité de Moléculas Nuevas emite opiniones favorables por unanimidad sobre remdesivir y vacuna Sinopharm \". www.gob.mx/cofepris (in Spanish).
- "Cofepris emite autorización para uso de emergencia a vacuna Sinopharm". www.gob.mx/cofepris (in Spanish).
- Cristina (19 March 2021). "O mie de studenți și medici-rezidenți din cadrul USMF vor fi imunizați anti-COVID cu vaccinul BBIBP-CorV, produs de către Sinopharm Beijing Institute of Biological Products". Ziarul de Gardă (in Romanian). Retrieved 19 March 2021.
- "Deputy PM and City Governor get the first dose of Sinopharm vaccine". Montsame. Retrieved 15 March 2021.
- "Montenegro receives 30,000 doses of China's COVID-19 vaccine". seenews. Retrieved 22 March 2021.
- "Mozambique expects to vaccinate 16 million against coronavirus by 2022". Reuters. 6 March 2021. Retrieved 7 March 2021.
- "Myanmar to use China-donated Sinopharm Covid vaccine". Eleven Media Group Co., Ltd. Retrieved 20 June 2021.
- Namibian T. "Khomas, Erongo first to get vaccinated". The Namibian. Archived from the original on 26 April 2021. Retrieved 17 March 2021.
- Poudel A. "China's Shinopharm vaccine gets emergency use authorisation in Nepal". Kathmandu Post. Retrieved 19 February 2021.
- "Nicaragua receives China vaccines after cutting ties with Taiwan". BBC News. 13 December 2021. Retrieved 4 March 2022.
- "China donates 400,000 doses of Sinopharm COVID-19 vaccine to Niger". CNA. Retrieved 1 April 2021.
- "Nigeria approves Sinopharm COVID vaccine, expects 7.7 mln doses". Reuters. 24 August 2021. Retrieved 28 August 2021.
- Son H, Ji J, Lee M (15 June 2021). "North Korea Has Been Importing Chinese COVID-19 Vaccines Since Early May". Radio Free Asia.
- "North Macedonia looks to Chinese vaccine to revive program". ABC News. Retrieved 1 May 2021.
- Shahzad A (19 January 2021). "Pakistan approves Chinese Sinopharm COVID-19 vaccine for emergency use". Reuters.
- "What we know about China's Sinopharm COVID vaccine". The Financial Express. Retrieved 1 May 2021.
- "Chinese vaccine to be gazetted". Post Courier. 7 May 2021. Retrieved 21 May 2021.
- "Peru grants 'exceptional' approval for Sinopharm COVID-19 vaccine – government sources". Reuters. 27 January 2021.
- Aguilar, Krissy (7 June 2021). "PH approves Sinopharm COVID-19 vaccine for emergency use". Philippine Daily Inquirer.
- "Rwanda to acquire Sinopharm vaccines next week". The New Times. Rwanda. 12 August 2021. Retrieved 28 August 2021.
- Asala K (18 February 2021). "Senegal Kicks Off COVID-19 Vaccination Campaign with China's Sinopharm". Africanews. Retrieved 23 February 2021.
- "Serbia Becomes First European Nation To Use China's Sinopharm Vaccine". Radio Free Europe/Radio Liberty. 20 January 2021.
- Thomas AR (15 March 2021). "Sierra Leone's President Bio leads the way in taking COVID-19 Vaccine". Sierra Leone Telegraph. Retrieved 16 March 2021.
- "11 Singapore private healthcare providers allowed to bring in Sinopharm COVID-19 vaccine". CNA. Retrieved 30 July 2021.
- "Health Ministry Prepares for Sinopharm Roll Out - Solomon Times Online". SolomonTimes. Retrieved 21 May 2021.
- "Somalia rolls out Sinopharm vaccines to boost fight against COVID-19". News Ghana. 15 April 2021. Retrieved 18 April 2021.
- "South Sudan to receive 100,000 doses of Sinopharm vaccines from China". Juba echo. 27 August 2021. Archived from the original on 29 August 2021. Retrieved 29 August 2021.
- "NMRA approves sinopharm vaccine for emergency use". Colombo Gazette. 19 March 2021. Retrieved 29 March 2021.
- "China To Provide Sudan With 250,000 Doses Of Sinopharm Vaccine On Friday – Ambassador". UrduPoint. Retrieved 14 April 2021.
- "StackPath". dailynewsegypt. 28 March 2021. Retrieved 14 April 2021.
- "FDA approves use of Sinopharm vaccine". Bangkok Post. 28 May 2021.
- "China's Sinopharm COVID-19 vaccine approved for marketing in Tunisia - Xinhua | English.news.cn". xinhuanet. Retrieved 21 July 2021.
- "В Туркменистане от COVID бесплатно прививают китайской вакциной, российский "Спутник" можно купить". Радио Азатлык (in Russian). 7 April 2021. Retrieved 9 May 2021.
- "PM to receive Sinopharm jab today". Vanuatu Daily Post. 15 June 2021. Retrieved 15 June 2021.
- Sequera V (1 March 2021). "Venezuela approves use of China's Sinopharm coronavirus vaccine". Reuters. Retrieved 2 March 2021.
- "Vietnam approves China's Sinopharm vaccine for use against COVID-19". Reuters. 4 June 2021. Retrieved 4 June 2021.
- Sachiti R. "Zambia to get Sinopharm COVID-19 vaccines". The Herald. Retrieved 21 May 2021.
- Mutsaka F (18 February 2021). "Zimbabwe starts administering China's Sinopharm vaccines". Toronto Star. Retrieved 20 February 2021.
- "COVID-19 Vaccine Update Supplement" (PDF). CARPHA.
- "Macau receives first batch of COVID-19 vaccines". IAG. 7 February 2021. Retrieved 24 February 2021.
- "WHO lists additional COVID-19 vaccine for emergency use and issues interim policy recommendations" (Press release). World Health Organization (WHO). 7 May 2021. Retrieved 7 May 2021.
- Taylor A (7 May 2021). "WHO grants emergency use authorization for Chinese-made Sinopharm coronavirus vaccine". The Washington Post. Retrieved 7 May 2021.
- "International COVID-19 vaccines recognised by Australia". Therapeutic Goods Administration (TGA). Retrieved 26 November 2021.
- "TGA recognises two more COVID-19 vaccines not registered in Australia but used widely internationally" (Press release). Therapeutic Goods Administration. 1 November 2021. Retrieved 11 November 2021.
- "Coronavirus: Entry Regulations to Austria". www.austria.info.
- Canada GA (8 April 2021). "COVID-19 vaccinated travellers entering Canada - Travel restrictions in Canada". travel.gc.ca.
- Bajón M. "Travel to Chile Plan".
- "Requisitos de entrada". Visit Costa Rica. 8 June 2016.
- "Vaccinated Passengers". Cyprus Flight Pass. Archived from the original on 6 March 2022. Retrieved 4 March 2022.
- Čeština (21 April 2021). "Recognition of vaccination certificates from other countries – Aktuální informace o COVID-19". Koronavirus.mzcr.cz. Retrieved 4 March 2022.
- "Vaccine series accepted in Finland - Infectious diseases and vaccinations - THL". Finnish Institute for Health and Welfare (THL), Finland. Archived from the original on 16 November 2021. Retrieved 16 November 2021.
- "Greece Accepting Most Covid-19 Vaccines for Travel". GTP Headlines. 11 August 2021.
- "Vaccinated Travellers – Ministry Of Health – Government of Grenada". Covid19.gov.gd. 6 November 2020. Archived from the original on 18 March 2022. Retrieved 4 March 2022.
- "Certificates of vaccination accepted at the border in Iceland for exemption of border measures due to COVID-19". www.landlaeknir.is. Archived from the original on 5 November 2021. Retrieved 5 November 2021.
- "Government advice on international travel". www.gov.ie. 14 July 2021. Archived from the original on 16 November 2021. Retrieved 16 November 2021.
- "Valstu saraksti" (in Latvian). SPKC. Retrieved 4 March 2022.
- "Protocolos COVID-19". tourismpanama.
- Arslan (1 August 2021). "Qatar gives conditional approval for Sinopharm Vaccine". qaziarslantariq.
- "Update vaccinated individuals (for the purpose of coming to the kingdom) December 2021 - Public Health Authority". Covid19.cdc.gov.sa. Retrieved 4 March 2022.
- "Border Crossing | GOV.SI". Portal GOV.SI.
- "Certificado Covid Digital De La Ue Y Otros Certificados" (PDF). Archived from the original (PDF) on 30 November 2021. Retrieved 16 November 2021.
- "NEW-FAQs". Saint Lucia Tourism Authority. Archived from the original on 8 December 2022. Retrieved 18 November 2021.
- "Certificate requirements for foreign nationals travelling to Sweden - The Public Health Agency of Sweden". www.folkhalsomyndigheten.se. Archived from the original on 16 November 2021. Retrieved 16 November 2021.
- FOPH FO. "Coronavirus: Entering Switzerland". www.bag.admin.ch. Archived from the original on 24 July 2020. Retrieved 12 November 2021.
- Zaken Mv (12 November 2021). "Travelling to the Netherlands with proof of vaccination - Coronavirus COVID-19 - Government.nl". www.government.nl.
- "Vaccinations for Turkey: Do I need an Approved COVID Vaccine?". 10 April 2019. Archived from the original on 16 November 2021. Retrieved 16 November 2021.
- "Visit Ukraine - Information and Rules". Visit Ukraine.
- "COVID-19 and Your Health". U.S. Centers for Disease Control and Prevention (CDC). 11 February 2020.
- "Requisitos para el ingreso a Uruguay de personas nacionales o extranjeras". Ministerio de Salud Pública.
- "An Open Study of the Safety, Tolerability and Immunogenicity of the Drug "Gam-COVID-Vac" Vaccine Against COVID-19". ClinicalTrials.gov. United States National Library of Medicine. 17 June 2020. NCT04436471. Retrieved 5 March 2021.
- Burki TK (November 2020). "The Russian vaccine for COVID-19". The Lancet. Respiratory Medicine. 8 (11): e85–e86. doi:10.1016/S2213-2600(20)30402-1. PMC 7837053. PMID 32896274.
- "Sputnik V vaccine granted full permanent approval in Russia". PR Newswire. 4 February 2022. Retrieved 8 February 2022.
- "Turkmenistan is the first in Central Asia to have registered 'Sputnik V' vaccine". Orient. 18 January 2021. Archived from the original on 27 January 2021. Retrieved 17 March 2021.
- "Session of the Cabinet of Ministers of Turkmenistan". Turkmenistan today. 22 January 2021.
- "Uzbekistan Certifies Russia's Sputnik Vaccine For Mass Use". Agence France-Presse (Barron's). 17 February 2021.
- "Covid19: National Pharmaceuticals Agency registers Sputnik V vaccine". Algeria Press service. 10 January 2021.
- "Angola, Congo Republic and Djibouti approve Russia's Sputnik V vaccine". Reuters. 3 March 2021.
- "Antigua and Barbuda authorizes Sputnik V". sputnikvaccine.com (Press release). 26 March 2021.
- "Argentina has registered the Sputnik V vaccine based on Russian clinical trial data" (Press release). Gamaleya Center. Retrieved 1 January 2021.
- "Armenia approves Russia's Sputnik V coronavirus vaccine -Russia's RDIF". Reuters. 1 February 2021. Retrieved 1 February 2021.
- "Russian Direct Investment Fund". rdif.ru. Retrieved 1 April 2021.
- "Bahrain authorises Sputnik V COVID-19 vaccine for emergency use – Bahrain TV". Reuters. 10 February 2021. Retrieved 19 February 2021.
- "Bangladesh approves Russia's Sputnik V Covid-19 vaccine for emergency use". Dhaka Tribune. 27 April 2021. Retrieved 27 April 2021.
- "Belarus registers Sputnik V vaccine, in first outside Russia – RDIF". Reuters. 21 December 2020. Retrieved 22 December 2020.
- "Ministerio de Salud de Bolivia – Bolivia y Rusia firman contrato para adquirir 5,2 millones de dosis de la vacuna Sputnik-V contra la COVID-19". minsalud.gob.bo. Retrieved 1 January 2021.
- "Sputnik V vaccine registered in Bosnia and Herzegovina's Republika Srpska". TASS. 5 February 2021. Retrieved 8 February 2021.
- "Anvisa aprova, com restrições, a importação excepcional de doses da Covaxin e Sputnik V" [Anvisa approves import of doses of Covaxin and Sputnik V, with restrictions]. G1 (in Portuguese). Globo. 4 June 2021. Retrieved 4 June 2021.
- "Russian Direct Investment Fund". rdif.ru. Archived from the original on 5 November 2021. Retrieved 5 November 2021.
- "Cameroon approves Russia's Sputnik V coronavirus vaccine for use – RDIF". Reuters. 19 March 2021.
- "Instituto de Salud Pública de Chile". Retrieved 21 July 2021.
- "Arcsa autoriza el ingreso al Ecuador de vacuna Sputnik V". www.controlsanitario.gob.ec. 14 May 2021. Retrieved 15 May 2021.
- "Sputnik V authorised in Gabon" (Press release). Gamaleya Center. Retrieved 17 February 2021.
- "Farmacovigilancia de vacunas para COVID-19 - Gamaleya Sputnik V; Sputnik Light". Farmacovigilancia de vacunas para COVID-19. Retrieved 9 October 2021.
- "Ghana approves Russia's Sputnik V vaccine for emergency use – RDIF". Reuters. 20 February 2021. Archived from the original on 19 March 2021. Retrieved 17 March 2021.
- "Guatemala authorizes Sputnik V". PharmiWeb. Retrieved 1 April 2021.
- "Guinea Begins Administering Russia's Sputnik V Covid-19 Vaccine". Africa news. 31 December 2020.
- "Russia's Sputnik V vaccine expands its reach in Latin America". CNN. 3 March 2021.
- "Honduras approves use of Sputnik V vaccine against COVID-19". Xinhuanet. 25 February 2021.
- "Hungarian drug regulator approves Sputnik V vaccine: website". The Moscow Times. 7 February 2021.
- "India approves Russia's 'Sputnik V' COVID-19 vaccine: Economic Times". Reuters. 12 April 2021.
- "BPOM Terbitkan Izin Darurat Vaksin Rusia Sputnik-V". CNN Indonesia (in Indonesian). 25 August 2021. Retrieved 25 August 2021.
- "Iran approves Russian coronavirus vaccine Sputnik V". Reuters. 26 January 2021.
- "Sputnik V authorized in Iraq" (Press release). PharmiWeb.com. 4 March 2021.
- "Jordan approves Russia's Sputnik V vaccine for use against COVID-19" (Press release). Reuters. 10 March 2021.
- "Kazakhstan begins mass vaccination by Russian Sputnik V". 1 February 2021. Retrieved 19 February 2021.
- "Morocco, Kenya approve Russian coronavirus vaccine for use – RDIF". 10 March 2021. Retrieved 12 March 2021.
- "Sputnik V registered in Kyrgyzstan". Gamaleya Center (Press release). 23 February 2021.
- "Lebanon authorises emergency use of Russia's Sputnik V vaccine". Reuters. 5 February 2021. Archived from the original on 19 March 2021. Retrieved 17 March 2021.
- "Maldives approves Russia's Sputnik V COVID-19 vaccine". reuters.com. 13 May 2021. Retrieved 13 May 2021.
- "Mali approves Russia's Sputnik V vaccine – Russian sovereign wealth fund". Reuters. 30 March 2021.
- "Mauritius approves Russia's Sputnik V coronavirus vaccine – Russian RDIF fund". Reuters. 22 March 2021.
- "Mexico, Germany warm to Russia's Sputnik V virus vaccine". The Jakarta Post. 3 February 2021.
- "Mongolia Approves Russia's Sputnik V Coronavirus Vaccine – RDIF". Urdu Point. 9 February 2021.
- "Montenegro and St. Vincent approve Russia's Sputnik V vaccine – RDIF". Reuters. 12 February 2021.
- "Morocco orders one million doses of Russia's Sputnik V vaccine". Yabiladi. 11 March 2021.
- "Myanmar registers Russia's Sputnik V COVID-19 vaccine". Tass. Retrieved 19 February 2021.
- "Russian Direct Investment Fund". rdif.ru. Retrieved 1 April 2021.
- "Nepal approves Russia's Sputnik V COVID-19 vaccine". Reuters. 20 April 2021. Retrieved 23 April 2021.
- "Nicaragua approves Russian COVID-19 vaccine". Associated Press. 3 February 2021. Retrieved 2 June 2021.
- "Sputnik V vaccine approved in Nigeria". Russian Direct Investment Fund. 15 July 2021. Archived from the original on 18 July 2021. Retrieved 18 July 2021.
- "COVID-19: Oman approves use of Sinovac and Sputnik vaccines". gulfnews. 24 June 2021. Retrieved 25 June 2021.
- "Govt okays Russian vaccine for 'emergency use'". Dawn. 24 January 2021.
- "Palestine has become the first country in the Middle East to register Sputnik V vaccine". RFID. 11 January 2021.
- "Panama approves use of Russian vaccine". Newsroom Panama. 1 April 2021. Retrieved 2 April 2021.
- "Paraguay approves Russia's Sputnik V vaccine: RDIF". Reuters. 15 January 2021. Retrieved 15 January 2021.
- "Perú anuncia acuerdo para compra de 20 millones de dosis de la vacuna Sputnik V". El Peruano. 20 July 2021. Retrieved 5 August 2021.
- "Russia's Sputnik V approved for emergency use in PH". CNN Philippines. 19 March 2021. Archived from the original on 19 March 2021. Retrieved 19 March 2021.
- "San Marino buys the Sputnik vaccine: "First doses already in the next few days"". Unioneonline. 20 February 2021. Archived from the original on 19 March 2021. Retrieved 17 March 2021.
- "San Marino reopens thanks to Sputnik covid vaccine from Russia". Wanted in Rome. 12 April 2021.
- "Agencija odobrila uvoz ruske vakcine Sputnjik V u Srbiju". N1 (in Serbian). 31 December 2020.
- "Sputnik V vaccine approved in Seychelles". TASS. 19 March 2021.
- "Sri Lanka approves Russia's Sputnik V vaccine". The Hindu. 4 March 2021.
- "Syria authorizes use of Sputnik-V". Roya. 22 February 2021.
- Mohammed O (17 June 2021). "Tanzania poised to join COVAX vaccine-sharing facility - WHO". Reuters. Retrieved 13 January 2022.
- "Sputnik V vaccine authorized in Tunisia" (Press release). Gamaleya Center. Retrieved 30 January 2021.
- Merve Aydogan (30 April 2021). "Turkey OKs emergency use of Sputnik-V vaccine". Anadolu Agency.
- "Erdogan unimpressed by Putin's COVID-19 antibodies, Sputnik V vaccine booster offer". Al Arabiya English. 29 September 2021. Retrieved 11 January 2022.
- "UAE approves Russia's Sputnik vaccine for emergency use". Khaleej Times. 21 January 2021. Retrieved 21 January 2021.
- "Venezuela firma contrato para la adquisición de la vacuna rusa Sputnik V" (in Spanish). Reuters. 29 December 2020.
- "Vietnam approves Russia Covid-19 vaccine for emergency use". VnExpress. Retrieved 2 April 2021.
- "Covid-19: Zimbabwe authorises Sputnik V, Sinovac vaccines for emergency use". news24.com. 9 March 2021.
- "Povolenie na terapeutické použitie neregistrovaného lieku" (PDF). 1 March 2021. Retrieved 8 July 2021.
- "Zmätky s vakcínami pokračujú. Aký Sputnik máme v skladoch?". Pravda.sk (in Slovak). 14 April 2021. Retrieved 8 July 2021.
- Update on the SAHPRA review of the Sputnik V vaccine. South African Health Products Regulatory Agency. 18 October 2021. Retrieved 20 April 2022.
- AfricaNews (20 October 2021). "South Africa rejects Russian-made coronavirus vaccine Sputnik V". Africanews. Retrieved 22 October 2021.
- "Vaccines we recognise for the purpose of travel to Australia". Therapeutic Goods Administration. Retrieved 17 January 2022.
- "Vaccinated/Recovered Passengers". Archived from the original on 16 November 2021. Retrieved 16 November 2021.
- "Questions and answers on coronavirus, related decisions and changes: Vaccines". kkk.kriis.ee. Retrieved 24 November 2021.
- "Greece Accepting Most Covid-19 Vaccines for Travel". GTP Headlines. 11 August 2021. Retrieved 20 November 2021.
- "Entry to Israel - Non-Israeli Citizens: Air Travel". Corona Traffic Light Model (Ramzor) Website. Retrieved 24 November 2021.
- "Travellers must have MySejahtera to enter Malaysia". themalaysianreserve.com. The Malaysian Reserve. 25 March 2022. Retrieved 25 March 2022.
- "Border Crossing". Portal GOV.SI. Retrieved 24 November 2021.
- "NEW-FAQs". Saint Lucia Tourism Authority. Archived from the original on 8 December 2022. Retrieved 24 November 2021.
- "THAILAND'S ENTRY REGULATIONS". Royal Thai Embassy in Vienna. Retrieved 20 December 2021.
- "Safety and Immunogenicity Study of Inactivated Vaccine for Prevention of SARS-CoV-2 Infection (COVID-19) (Renqiu)". ClinicalTrials.gov. United States National Library of Medicine. 12 May 2020. NCT04383574. Archived from the original on 11 October 2020. Retrieved 14 July 2020.
- "Clinical Trial of Efficacy and Safety of Sinovac's Adsorbed COVID-19 (Inactivated) Vaccine in Healthcare Professionals (PROFISCOV)". ClinicalTrials.gov. United States National Library of Medicine. 2 July 2020. NCT04456595. Archived from the original on 11 October 2020. Retrieved 3 August 2020.
- PT. Bio Farma (10 August 2020). "A Phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-COV-2 inactivated vaccine in healthy adults aged 18–59 years in Indonesia". Registri Penyakit Indonesia. Retrieved 15 August 2020.
- Liu R (6 February 2021). "China approves Sinovac Biotech COVID-19 vaccine for general public use". Reuters. Retrieved 7 February 2021.
- "Use of Sinovac vaccine authorised". Government of Hong Kong. 18 February 2021. Retrieved 19 February 2021.
- "В Армению отправлена китайская вакцина от COVID-19". Общественное Радио Армении (in Russian). Retrieved 9 May 2021.
- Aliyev J. "Azerbaijan kicks off COVID-19 vaccination". Anadolu Agency. Retrieved 7 February 2021.
- "Bangladesh approves Sinovac Covid-19 vaccine for emergency use". Dhaka Tribune. 6 June 2021. Retrieved 6 June 2021.
- Ayosso A (29 March 2021). "Deux ministres vaccinés contre Covid-19". 24haubenin.info (in French). Retrieved 7 April 2021.
- "Bolívia autoriza uso de vacinas Sputnik V e CoronaVac contra covid-19". noticias.uol.com.br (in Brazilian Portuguese). Retrieved 6 January 2021.
- Agency A (28 March 2021). "Turkey sends Chinese COVID-19 vaccines to Bosnia-Herzegovina". Daily Sabah. Retrieved 14 April 2021.
- "Botswana Could Be First African Country to Vaccinate Entire Adult Population". Voice of America. 27 April 2021. Retrieved 21 May 2021.
- McGeever J, Fonseca P (17 January 2021). "Brazil clears emergency use of Sinovac, AstraZeneca vaccines, shots begin". Reuters. Retrieved 17 January 2021.
- Chanritheara T. "Cambodia Approves AstraZeneca and Sinovac Vaccines for COVID-19 Emergency Use". Cambodianess. Retrieved 12 February 2021.
- "Chile aprueba el uso de emergencia de la vacuna china de Sinovac contra covid-19". France 24. 20 January 2021. Retrieved 30 January 2021.
- Aliyev J. "Colombia approves emergency use of CoronaVac vaccine". Anadolu Agency. Retrieved 7 February 2021.
- "Djibouti Ministry of Health". Twitter. Retrieved 30 March 2021.
- "Anticovid vaccines run out as Dominican Republic awaits arrival of more doses". DominicanToday. Retrieved 10 March 2021.
- Martins F. "Government to launch Chinese Sinovac vaccine next week". TATOLI Agência Noticiosa de Timor-Leste. Retrieved 15 June 2021.
- "Ecuador signs agreement with Sinovac for 2 million COVID-19 vaccine: minister". National Post. Retrieved 26 February 2021.
- "Egypt approves Sinovac COVID-19 vaccine for emergency use". gulfnews. 27 April 2021. Retrieved 27 April 2021.
- "Llegan a El Salvador un millón de dosis de la vacuna china CoronaVac contra el covid-19 de la farmacéutica Sinovac". La Prensa Gráfica (in European Spanish). Retrieved 29 March 2021.
- "China to donate Sinovac Vaccine to Fiji". Fiji Broadcasting Corporation. Retrieved 22 March 2021.
- "Covid-19 vaccination with Sinovac to start on May 24 in Georgia". Agenda.ge. Retrieved 21 May 2021.
- "Guinea receives purchase of 300,000 Sinovac COVID-19 vaccines". CNA. Retrieved 3 June 2021.
- Soeriaatmadja W (11 January 2021). "Indonesia grants emergency use approval to Sinovac's vaccine, local trials show 65% efficacy". The Straits Times. Retrieved 11 January 2021.
- "BPOM Grants Emergency Use Authorization for Sinovac Vaccine". Tempo. 11 January 2021. Retrieved 11 January 2021.
- "В Казахстан доставили первую партию китайской вакцины CoronaVac". Kazakhstan Today (in Russian). Retrieved 7 June 2021.
- "Libya begins administrating China's Sinovac vaccine in Tripoli". Libyan Express. 9 May 2021. Retrieved 21 May 2021.
- "Malaysia approves Sinovac, AstraZeneca COVID-19 vaccines for use". Reuters. 2 March 2021. Retrieved 21 November 2021.
- Barrera A (11 February 2021). "Mexico approves China's CanSino and Sinovac COVID-19 vaccines". Reuters. Retrieved 11 February 2021.
- Tanas A (9 April 2021). "Moldova to buy 400,000 doses of Sinovac COVID-19 vaccine". Reuters. Retrieved 9 May 2021.
- Fishbein E. "Ethnic groups step in as Myanmar's COVID response falls apart". Al Jazeera. Retrieved 23 July 2021.
- "Nepal gives emergency use approval to China's Sinovac Covid-19 vaccine". kathmandupost. Retrieved 7 June 2021.
- Testorides K (27 June 2021). "North Macedonia gets 500,000 doses of Sinovac vaccines". Associated Press. Retrieved 28 June 2021.
- "DRAP allows emergency authorisation to fifth Covid-19 vaccine". The News International. Retrieved 10 April 2021.
- "Panama approves emergency use of Chinese vaccine against COVID-19". Xinhuanet. 9 April 2021. Retrieved 9 April 2021.
- "CoronaVac, vacuna de alta eficacia". Ministerio de Salud Publica Y Bienestar Social (Paraguay).
- "Philippines approves Sinovac's COVID-19 vaccine for emergency use". Reuters. 22 February 2021.
- "MoH: Six COVID-19 vaccines approved in Saudi Arabia". Saudi Gazette. 24 August 2021. Retrieved 25 December 2021.
- Matthew Mohan (23 October 2021). "Sinovac COVID-19 vaccine to be included in Singapore's national vaccination programme". CNA. Retrieved 23 October 2021.
- "SAHPRA authorises the CoronaVac vaccine with conditions". SAHPRA. 3 July 2021.
- "Sri Lanka approves China's Sinovac COVID-19 vaccine for emergency use". ColomboPage. Retrieved 18 July 2021.
- Китайской вакциной в Таджикистане первоначально будут прививать людей старше 60 лет. ASIA-Plus. Retrieved 13 July 2021.
- "Vaccine-Holdout Tanzania Begins Inoculations With Sinovac Doses - BNN Bloomberg". Bloomberg. 13 July 2021. Retrieved 13 July 2021.
- Thepgumpanat P (22 February 2021). "Thailand allows emergency use of Sinovac's COVID-19 vaccine". Reuters. Retrieved 23 February 2021.
- "Le CoronaVac pour les étudiants du public". République Togolaise (in French). Retrieved 23 May 2021.
- "Health Ministry: Covaxin vaccine approved use in Trinidad and Tobago". Trinidad and Tobago Newsday. 8 November 2021. Retrieved 30 November 2021.
- "Tunisia approva vaccino cinese Sinovac" (in Italian). Agenzia Nazionale Stampa Associata (in Italian). 5 March 2021. Retrieved 7 March 2021.
- "Turkey grants emergency authorization to Sinovac's CoronaVac". Reuters. 14 January 2021. Retrieved 9 January 2022.
- turkmenportal. "В Туркменистан поступила крупная партия китайской вакцины "CoronaVac"". Туркменистан, интернет портал о культурной, деловой и развлекательной жизни в Туркменистане (in Russian). Retrieved 7 June 2021.
- Zinets N (9 March 2021). "Ukraine approves China's Sinovac COVID-19 vaccine". Reuters. Retrieved 10 March 2021.
- "Uzbekistan plans to obtain Sinovac coronavirus vaccine". akipress. Retrieved 21 May 2021.
- "COVID-19 Information". U.S. Embassy in Venezuela. 30 June 2021. Retrieved 23 August 2021.
- Nebehay S (1 June 2021). "WHO approves Sinovac COVID vaccine, the second Chinese-made dose listed". Reuters. Geneva. Retrieved 1 June 2021.
- "WHO recommendation Sinovac COVID-19 vaccine (Vero Cell [Inactivated]) – CoronaVac". World Health Organization (WHO). 1 May 2021. Archived from the original on 2 June 2021. Retrieved 1 June 2021.
- "WHO validates Sinovac COVID-19 vaccine for emergency use and issues interim policy recommendations". World Health Organization (WHO) (Press release). Retrieved 1 June 2021.
- "Australia set to restart international travel in November, Scott Morrison says". The Guardian. 1 October 2021. Retrieved 21 November 2021.
- "CInternational travels to Norway". 23 March 2020.
- "Travelers Vaccinations - Ministry of Health and Prevention - UAE". www.mohap.gov.ae. Archived from the original on 22 November 2021. Retrieved 22 November 2021.
- "Việt Nam temporarily approves COVID-19 vaccine certificates by 72 countries, territories: Foreign ministry". Việt Nam News. 21 October 2021. Retrieved 8 January 2022.
- "WHO lists 9th COVID-19 vaccine for emergency use with aim to increase access to vaccination in lower-income countries". World Health Organization (WHO) (Press release). 17 December 2021. Retrieved 30 October 2022.
- "WHO lists 10th COVID-19 vaccine for emergency use: Nuvaxovid". World Health Organization (WHO) (Press release). 21 December 2021. Retrieved 30 October 2022.
- "TGA provisionally approves Novavax (Biocelect Pty Ltd's) COVID-19 vaccine NUVAXOVID". Therapeutic Goods Administration. 20 January 2022. Retrieved 20 January 2022.
- "Novavax Nuvaxovid COVID-19 vaccine". Health Canada. 7 June 2022. Retrieved 30 October 2022.
- "Canada approves Novavax's COVID-19 vaccine for adults". Reuters. 17 February 2022. Retrieved 17 February 2022.
- "Japan approves Novavax as 4th COVID vaccine amid new surge". ABC News. 19 April 2022. Retrieved 21 April 2022.
- "South Korea Ministry of Food and Drug Safety Approves Novavax COVID-19 Vaccine". Novavax, Inc. 12 January 2022. Retrieved 22 January 2022.
- "Novavax Reports First Quarter 2022 Financial Results and Operational Highlights". Novavax, Inc. 9 May 2022.
- Achom D (28 December 2021). "India Clears 2 New Vaccines And Merck's Covid Pill". NDTV.com.
- O'donnell C, Nadeem D (1 November 2021). "Novavax COVID-19 vaccine gets first authorization; expects more within weeks, CEO says". Reuters. Retrieved 1 November 2021.
- The Associated Press Staff (3 February 2022). "Novavax's COVID-19 shot receives provisional approval in New Zealand". Associated Press. Retrieved 4 February 2022.
- "Philippines approves emergency use of Novavax's COVID-19 vaccine". Reuters. 17 November 2021. Retrieved 17 November 2021.
- Clara Chong (14 February 2022). "Novavax Covid-19 vaccine approved for those aged 18 and above in Singapore". The Straits Times. Retrieved 14 February 2022.
- "Swissmedic grants temporary authorisation for the Nuvaxovid COVID-19 vaccine from Novavax". Swissmedic. 13 April 2022.
- "Novavax cleared for use by Taiwan FDA". Taiwan News. 19 June 2022. Retrieved 24 June 2022.
- "Novavax COVID-19 vaccine Nuvaxovid approved by MHRA". GOV.UK. Retrieved 3 February 2022.
- "Coronavirus (COVID-19) Update: FDA Authorizes Emergency Use of Novavax COVID-19 Vaccine, Adjuvanted" (Press release). U.S. Food and Drug Administration. 13 July 2022. Retrieved 14 July 2022.
- Drillinger M (13 July 2022). "FDA Authorizes Novavax's COVID-19 Vaccine—Here's What to Know About the New Shot". Health. Retrieved 14 July 2022.
- "EMA recommends Nuvaxovid for authorisation in the EU". European Medicines Agency (EMA). 20 December 2021.
- "COVID-19 Vaccine Update Supplement, Week of 13th – 17th June, 2022" (PDF). CARPHA. p. 9. Retrieved 20 November 2022.
- "WHO lists 9th COVID-19 vaccine for emergency use with aim to increase access to vaccination in lower-income countries". World Health Organization (WHO) (Press release). Retrieved 17 December 2021.
- "WHO issues emergency use listing to Novavax-Serum Institute's COVID-19 vaccine". Reuters. 17 December 2021.
- "Bahrain authorizes COVAXIN for emergency use". Bahrain News Agency. 11 November 2021. Retrieved 29 January 2022.
- "Covid-19: Bharat Biotech ramps up Covaxin capacity to 700 million doses per annum | India News". The Times of India. 20 April 2021.
- "COVID-19 vaccine doses administered in the Americas". ais.paho.org.
- "Iran issues permit for emergency use for three other COVID-19 vaccines: Official". IRNA English. 17 February 2021.
- "Covaxin given conditional approval under emergency use protocols". nst.com.my. New Straits Times. 10 February 2021. Retrieved 10 February 2022.
- Livemint (1 November 2021). "5 more countries recognise India's Covid vaccination certificate. Details here". mint. Retrieved 1 November 2021.
- "México autoriza Covaxin, la vacuna india contra covid-19". Excelsior. 6 April 2021.
- Sharma G (19 March 2021). "Nepal becomes third country to give emergency nod to Indian vaccine COVAXIN". Reuters. Retrieved 19 March 2021.
- "India provides 100,000 doses of Covaxin vaccine to Paraguay". Hindustan Times. 30 March 2021.
- "Philippine FDA issues full EUA to Bharat Biotech's Covaxin". Manila Bulletin. 23 June 2021.
- "FDA grants full emergency use authorization for Covaxin". CNN Philippines. 25 June 2021. Archived from the original on 3 July 2021. Retrieved 25 June 2021.
- "Covid-19: Hong Kong, Vietnam latest to approve Covaxin for emergency use". Hindustan Times. 10 November 2021.
- "Zimbabwe approves Covaxin, first in Africa to okay India-made Covid-19 vaccine". Hindustan Times. 4 March 2021. Retrieved 3 July 2021.
- "India's first homegrown COVID-19 shot wins WHO emergency use listing". Reuters. 3 November 2021.
- "WHO issues emergency use listing for eighth COVID-19 vaccine". World Health Organization (WHO) (Press release). Retrieved 3 November 2021.
- "Thailand Pass FAQs". Department of Consular Affairs. Retrieved 11 January 2022.
- "Valneva receives EUA from UAE for its inactivated COVID-19 vaccine". Express Pharma. 17 May 2022. Retrieved 20 June 2022.
- "COVID-19 Vaccine (inactivated, adjuvanted) Valneva EPAR". European Medicines Agency (EMA). 20 June 2022. Archived from the original on 28 June 2022. Retrieved 28 June 2022.
- Kansteiner F (10 November 2022). "Sanofi, GSK crash the COVID-19 vaccine party late with a world-first nod for their next-gen booster". Fierce Pharma. Retrieved 18 November 2022.
- Ryumin A (20 February 2021). "Russia registers its third COVID-19 vaccine CoviVac". Moscow: TASS. Retrieved 6 March 2021.
- "Single dose vaccine, Sputnik Light, authorized for use in Russia" (Press release). Russian Direct Investment Fund. 6 May 2021. Retrieved 11 May 2021.
- "Sputnik Light Vaccine Approved For Use In Angola". Sputnik V vaccine. 12 May 2021. Retrieved 13 May 2021.
- "BOLETIN OFICIAL REPUBLICA ARGENTINA - MINISTERIO DE SALUD - Resolución 3451/2021". www.boletinoficial.gob.ar. Retrieved 7 December 2021.
- "Armenia approves Russian one-dose Sputnik Light vaccine". Public Radio of Armenia. Retrieved 8 December 2021.
- "Bahrain authorizes emergency use of one-shot Sputnik-Light COVID-19 vaccine". Reuters. 11 May 2021. Retrieved 12 May 2021.
- "Belarus registers Russia's Sputnik Light vaccine for use". 3 June 2021. Retrieved 4 June 2021.
- "Sputnik Light vaccine authorized in Benin, says Russian Direct Investment Fund". TASS. 14 January 2022. Retrieved 7 February 2022.
- "Single-dose Sputnik Light vaccine approved for use in the Republic of the Congo". rdif.ru. Russian Direct Investment Fund. 7 June 2021. Archived from the original on 7 June 2021. Retrieved 7 June 2021.
- "The single-component Sputnik Light vaccine authorized in Egypt". sputnikvaccine. Retrieved 1 October 2021.
- "Sputnik Light: DCGI nod to emergency use of single-dose Covid vaccine". Livemint. 2 February 2022.
- "Kazakhstan approves Russia's single-dose Sputnik Light vaccine". Reuters. Moscow. 15 July 2021. Retrieved 15 July 2021.
- "Single-dose Sputnik Light vaccine registered in Kyrgyzstan". sputnikvaccine. Retrieved 25 June 2021.
- "Russia's Sputnik Light vaccine approved for use in Mauritius". Reuters. 2 June 2021. Retrieved 4 June 2021.
- "Single-dose Sputnik Light vaccine registered in Mongolia" (Press release). Moscow: Russian Direct Investment Fund. 11 June 2021. Archived from the original on 29 June 2021. Retrieved 25 June 2021.
- "Single-dose Sputnik Light vaccine approved for use in Nicaragua". Russian Direct Investment Fund. 20 May 2021. Archived from the original on 3 June 2021. Retrieved 1 June 2021.
- "Russia's Sputnik Light vaccine approved for use in Palestinian territories". Reuters. 31 May 2021. Retrieved 4 June 2021.
- "Philippines approves emergency use of Russia's Sputnik Light vaccine". Reuters. 23 August 2021. Retrieved 23 August 2021.
- "Russia Approves Single-Dose Sputnik Light Covid Vaccine For Use". NDTV Coronavirus. 6 May 2021.
- "Russian Direct Investment Fund". rdif.ru. Archived from the original on 30 November 2021. Retrieved 30 November 2021.
- "Batch of Russia's Sputnik Light vaccine delivered to Syria". TASS. Damascus. 24 July 2021. Retrieved 31 July 2021.
- "UAE authorises Sputnik Light COVID-19 vaccine -Russia's RDIF". Reuters. 6 October 2021. Retrieved 23 October 2021.
- "Single-dose Sputnik Light vaccine approved for use in Venezuela" (Press release). Gamaleya Center. Retrieved 15 May 2021.
- "Moscow Plays Up Donbas As Part Of The Ukraine Conflict". Warsaw Institute. 30 July 2021. Retrieved 4 March 2022.
- Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, et al. (August 2020). "Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial". Lancet. 396 (10249): 479–88. doi:10.1016/s0140-6736(20)31605-6. PMC 7836858. PMID 32702299.
- "China approves two more domestic COVID-19 vaccines for public use". Reuters. 25 February 2021. Retrieved 16 March 2021.
- "Argentina issues emergency approval to China's single-dose Cansino COVID-19 vaccine". Reuters. 11 June 2021. Retrieved 11 June 2021.
- "ISP Approves Emergency Use And Importation Of Cansino Vaccine To Fight COVID-19". Institute of Public Health of Chile. Retrieved 8 April 2021.
- "Ecuador authorizes use of China's CanSino vaccine against COVID-19". Reuters. 15 June 2021. Retrieved 16 June 2021.
- "China's CanSino Biologics COVID-19 vaccine receives emergency use approval in Hungary". MSN. 22 March 2021. Retrieved 22 March 2021.
- Shahzad A (12 February 2021). "Pakistan approves Chinese CanSinoBIO COVID vaccine for emergency use". Reuters. Retrieved 12 February 2021.
- "WHO validates 11th vaccine for COVID-19". World Health Organization (Press release). 19 May 2022. Retrieved 30 October 2022.
- Wee SL (25 February 2021). "China approves two more Covid-19 vaccines". The New York Times. Retrieved 1 April 2021.
- De Vera, Analou (25 August 2021). "FDA grants EUA to COVID-19 vaccine Sinopharm manufactured by Wuhan affiliate". Manila Bulletin.
- "COVID: Cuba approves emergency use of own Abdala vaccine". Deutsche Welle. 9 July 2021. Retrieved 9 July 2021.
- "Aprueba el CECMED el Autorizo de Uso de Emergencia del candidato vacunal cubano ABDALA". CECMED (in Spanish). Retrieved 9 July 2021.
- "Cofepris emite autorización para uso de emergencia de vacuna Abdala". www.gob.mx/cofepris (in Spanish). 29 December 2021. Retrieved 29 December 2021.
- "Nicaragua authorises two COVID-19 vaccines from Cuba, Cuban firm says". Reuters. 2 October 2021. Retrieved 4 October 2021.
- "Vincentians have access to Cuban Abdala vaccine". Loop News. Retrieved 28 December 2021.
- "St Vincent, The Grenadines to use Cuba's Abdala vaccine". Political Lore. 28 December 2021. Retrieved 13 January 2022.
- "Venezuela receives first shipment of Cuban coronavirus vaccine". Reuters. 25 June 2021. Retrieved 3 July 2021.
- "Conozca las 20 vacunas aceptadas para el ingreso de viajeros a Colombia". www.cali.gov.co (in Spanish). Retrieved 15 December 2021.
- "Requirements for Port Entry into Guyana" (PDF). www.health.gov.gy/index.php/component/k2/item/304-travel-guidelines. November 2021. Retrieved 2 March 2022.
- "Travel Restrictions Entering Guyana from Cuba". airheart.com/travel-bans. 19 January 2022. Archived from the original on 2 March 2022. Retrieved 2 March 2022.
- "Study of the Safety, Reactogenicity and Immunogenicity of "EpiVacCorona" Vaccine for the Prevention of COVID-19 (EpiVacCorona)". ClinicalTrials.gov. United States National Library of Medicine. 22 September 2020. NCT04368988. Retrieved 16 November 2020.
- "Turkmenistan registers vaccines for the prevention of infectious diseases". Turkmenistan Today. 29 January 2021.
- "Russian vaccine "EpiVacCorona" was registered in Turkmenistan". EN24. 29 January 2021. Archived from the original on 19 March 2021. Retrieved 17 March 2021.
- "Belarus receives Russia's EpiVacCorona vaccine". Belta. 3 February 2021. Retrieved 5 April 2021.
- "О регистрации вакцины ФБУН ГНЦ ВБ "Вектор" Роспотребнадзора "ЭпиВакКорона"". Rospotrebnadzor (in Russian). 14 October 2020. Archived from the original on 1 November 2020. Retrieved 17 March 2021.
- "Russia's EpiVacCorona vaccine post-registration trials started". The Pharma Letter. 18 November 2020.
- "COVID-19 vaccine development pipeline (Refresh URL to update)". Vaccine Centre, London School of Hygiene and Tropical Medicine. 1 March 2021. Retrieved 10 March 2021.
- Liu R (15 March 2021). "China IMCAS's COVID-19 vaccine obtained emergency use approval in China". Reuters. Retrieved 15 March 2021.
- "Invima recomienda el uso de emergencia de Zifivax, otra vacuna anticovid producida en China". infobae (in European Spanish). 27 January 2022. Retrieved 24 June 2022.
- "BPOM Setujui Penggunaan Dalam Kedaruratan Vaksin Zifivax". Republika.co.id (in Indonesian). 7 October 2021. Retrieved 7 October 2021.
- "Pakistan gears up for China's "ZF-2001 vaccine"--China Economic Net". en.ce.cn. Retrieved 30 November 2021.
- Mamatkulov M (1 March 2021). "Uzbekistan approves Chinese-developed COVID-19 vaccine". Reuters. Retrieved 2 March 2021.
- "Autorizo de emergencia SOBERANA 02 en Irán". finlay.edu.cu. Instituto Finlay de Vacunas. 1 July 2021. Retrieved 6 July 2021.
- "Cuba grants emergency approval to second homegrown COVID-19 vaccine". GMA News. 21 August 2021.
- "Nicaragua authorises two COVID-19 vaccines from Cuba, Cuban firm says". Reuters. 2 October 2021.
- "Iran and Hungary to Mutually Honour Immunity Certificates". Hungary Today. 16 December 2021. Retrieved 19 December 2021.
- "Briefing with Deputy Prime Minister Tatyana Golikova, Health Minister Mikhail Murashko and Head of Rospotrebnadzor Anna Popova". Government of Russia. 18 January 2021. Retrieved 20 February 2021.
- "Минздрав выдал разрешение на ввоз и применение вакцины КовиВак" (in Russian). Ministry of Health of the Republic of Belarus. 16 December 2021.
- "Russia approves its third COVID-19 vaccine, CoviVac". Reuters. 20 February 2021.
- "A new vaccine on the scene: Kazakhstan begins rollout of homegrown QazVac". Fortune. 26 April 2021.
- "Vaccination against COVID-19: QazVac registered in Kyrgyzstan". 24.kg. 19 August 2021.
- "Kyrgyzstan to start using Kazakh-made QazVac after expert review". Eureporter. 19 August 2021.
- "The Covid-19 Vaccine Qazvac Is Firstly Registrated[sic] Abroad". Biosafety.kz. 18 August 2021.
- "Kangtai Biological's COVID-19 vaccine gets emergency use approval in China". Reuters. 14 May 2021. Retrieved 20 May 2021.
- Shenzhen Kangtai Biological Products Co., Ltd. "BioKangtai's adenovirus vector COVID-19 vaccine obtained EUA in Indonesia". PR Newswire (Press release). Retrieved 30 November 2021.
- "Taiwan approves Medigen's COVID-19 vaccine candidate". Reuters. 19 July 2021. Retrieved 19 July 2021.
- "Paraguay aprueba vacuna taiwanesa Medigen para uso de emergencia". Diario HOY. 14 February 2022.
- "Taiwan to donate domestic COVID-19 vaccines to Somaliland". Focus Taiwan. 28 December 2021. Retrieved 7 January 2022.
- "MOHW Approves Entry for Persons with the Taiwanese Medigen Jab". Love FM News. 30 November 2021. Archived from the original on 7 January 2022. Retrieved 8 January 2022.
- "Negara yang mengakui vaksin Medigen menjadi 4 negara". PTS news. 16 November 2021. Retrieved 8 January 2022.
- "COVID-19 Pandemic Entry Requirements – PalauGov.pw". Retrieved 24 December 2021.
- Raghavan P (10 June 2021). "Explained: How is Biological E's Corbevax different?". The Indian Express. Retrieved 29 December 2021.
- "Botswana Approves Corbevax Covid Vaccine, Plans Local Output". Bloomberg.com. 28 March 2022. Retrieved 29 June 2022.
- "Iran issues license on its coronavirus vaccine". Trend.Az. 14 June 2021. Retrieved 14 June 2021.
- "Nicaragua recibe 200 mil dosis de la vacuna Coviran donadas por Irán". El 19 Digital (in Spanish). Retrieved 12 July 2022.
- "Iran and Hungary to Mutually Honour Immunity Certificates". Hungary Today. 16 December 2021. Retrieved 18 December 2021.
- "Vaccinations for Turkey: Do I need an Approved COVID Vaccine?". Turkey Visa. 10 April 2019. Archived from the original on 16 November 2021. Retrieved 26 December 2021.
- Liu R, Woo R (9 June 2021). "China builds new plant for IMBCAMS COVID-19 vaccine -state media". Reuters. Retrieved 15 June 2021.
- "India gives emergency approval for world's first COVID-19 DNA vaccine". Reuters. 20 August 2021.
- "Iran Authorizes Emergency Use of Third Homegrown Vaccine - Defense news". Tasnim News Agency. 9 September 2021. Archived from the original on 9 September 2021. Retrieved 9 September 2021.
- "Iran issues emergency permit for new local Covid-19 vaccine". Mehr News Agency. 6 October 2021. Retrieved 12 October 2021.
- "Two homegrown vaccines receive emergency use license". Tehran Times. 1 November 2021. Retrieved 27 November 2021.
- Aliyev J, Nur Duz Z (22 December 2021). "Turkey's domestic COVID-19 vaccine Turkovac approved for emergency use". Anadolu Agency. Retrieved 26 December 2021.
- "Emergency use of Sinopharm's new CNBG protein vaccine approved in UAE". Gulf News. 27 December 2021. Retrieved 27 December 2021.
- Dönmez BB (21 August 2021). "Cuba grants emergency use to 2 virus vaccines". Anadolu Agency. Retrieved 23 September 2021.
- "Cuban COVID-19 vaccine registered in Belarus". eng.belta.by. 27 July 2022. Retrieved 27 July 2022.
- Dönmez BB (21 August 2021). "Cuba grants emergency use to 2 virus vaccines". Anadolu Agency. Retrieved 23 September 2021.
- "Medicago Covifenz COVID-19 vaccine". Health Canada. 7 June 2022. Retrieved 30 October 2022.
- ""Noora" vaccine receives emergency use license". Tehran Times. 1 March 2022. Retrieved 2 March 2022.
- Kim TH (29 June 2022). "South Korea approves first homemade COVID-19 vaccine". Associated Press. Retrieved 29 June 2022.
- "SARS-CoV-2 mRNA vaccine". go.drugbank.com. Retrieved 10 April 2021.
- Widianto S, Liu R (30 September 2022). "A Chinese mRNA COVID vaccine is approved for the first time - in Indonesia". Reuters. Retrieved 18 November 2022.
- "Emergency-use approval for Bharat Biotech's iNCOVACC intranasal vaccin". www.thepharmaletter.com. 14 September 2022. Retrieved 20 November 2022.
- Haseltine WA. "Self-Amplifying mRNA Vaccine Receives EUA Nod From Indian Regulators". Forbes. Retrieved 20 November 2022.
- "Livzon Pharma's COVID vaccine gets approval as booster in China". Reuters. 2 September 2022. Retrieved 20 November 2022.