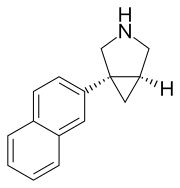
EB-1020
Centanafadine
Serotonin-norepinephrine-dopamine reuptake inhibitor
Centanafadine (INN) (former developmental code name EB-1020) is a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI) that began its development with Euthymics Bioscience after they acquired DOV Pharmaceutical. It was developed as a treatment for attention-deficit hyperactivity disorder (ADHD) and inhibits the reuptake of norepinephrine, dopamine, and serotonin with a ratio of 1:6:14, respectively.[1][2][3][4] In 2011, Euthymics Bioscience spun off its development of centanafadine to a new company called Neurovance.[5][6] In March 2017, Otsuka Pharmaceutical acquired Neurovance and the rights to centanafadine.[7] As of January 2018, Otsuka's pipeline indicates it is in Phase II and III clinical trials for a number of different applications to medical conditions.[8][9][10]